The market situation for 4-Methylamphetamine (4-MA) reflects its status as a designer drug and research chemical, albeit with limited availability and a niche presence. While it’s not as prevalent as other substances, 4-MA is occasionally sold by online vendors and sellers catering to those interested in exploring novel compounds.
As a designer drug and research chemical, 4-MA may attract individuals seeking unique psychoactive experiences or those engaged in scientific investigations. Sellers advertising 4-MA for sale often operate in a legal grey area, as the substance’s legality can vary widely by jurisdiction.
Potential buyers interested in acquiring 4-MA should carefully research its legal status in their region. Additionally, they should prioritize safety and responsible use due to the limited information about its effects and potential risks.
In summary, 4-methylamphetamine occupies a relatively small corner of the designer drug and research chemical market. Online vendors may occasionally offer it for sale to a select clientele. However, potential users should exercise caution, adhere to local regulations, and prioritize harm-reduction practices if they choose to explore this substance.
Contents
- 1 Summary
- 2 Legal status
- 3 FAQ
- 3.1 1. What is 4-Methylamphetamine (4-MA)?
- 3.2 2. What are the primary effects of 4-MA?
- 3.3 3. Is 4-MA legal?
- 3.4 4. How is 4-MA typically consumed?
- 3.5 5. What is its history and research background?
- 3.6 6. Are there any safety concerns associated with 4-MA use?
- 3.7 7. Can 4-MA be detected in drug tests?
- 3.8 8. What should I do if I or someone I know experiences adverse effects from 4-MA use?
- 3.9 9. What is the risk associated with the consumption of 4-MA-contaminated substances?
- 4 References
Summary
4-Methylamphetamine (4-MA), or PAL-313, Aptrol, or p-TAP, belongs to the phenethylamine and amphetamine chemical classes, functioning as a stimulant and anorectic drug.
In laboratory settings, it demonstrates potent and well-balanced activity as a serotonin, norepinephrine, and dopamine-releasing agent, with affinity values of 53.4nM, 22.2nM, and 44.1nM at the respective transporters. However, recent in vivo studies employing microdialysis in rats have revealed a different pattern. These studies indicate that 4-methylamphetamine significantly elevates serotonin levels (~18 times baseline) compared to dopamine (~5 times baseline). This discrepancy may stem from serotonin release inhibiting dopamine release, possibly through the activation of 5-HT2C receptors or the subsequent encouragement of GABA release, which exerts an inhibitory influence on dopamine neurons.
In 1952, 4-MA was explored as an appetite suppressant and even assigned the trade name Aptrol. However, its development appears to have been abandoned. More recently, it has resurfaced as a novel designer drug.
In animal experiments, 4-MA has shown the lowest self-administration rate among similar drugs tested, including 3-methylamphetamine, 4-fluoroamphetamine, and 3-fluoroamphetamine. This likely stems from its heightened potency in releasing serotonin relative to dopamine.
It’s important to note that 4-methylamphetamine was linked to a series of unfortunate incidents in Europe between 2012 and 2013, where several deaths were reported due to the consumption of amphetamine (‘speed’) contaminated with this substance.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 64-11-9 |
|---|---|
| PubChem CID | 199116 |
| ChemSpider | 172349 |
| UNII | 9E273KL7HS |
| ChEMBL | ChEMBL166183 |
| CompTox Dashboard (EPA) | DTXSID50945351 |
| Chemical and physical data | |
| Formula | C10H15N |
| Molar mass | 149.237 g·mol−1 |
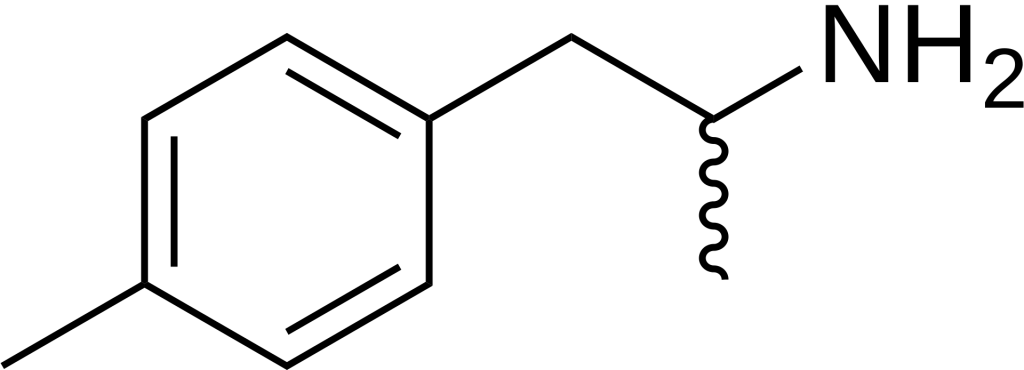
Legal status
| Legal status | |
|---|---|
| Legal status | CA: Schedule I DE: Anlage II (Authorized trade only, not prescriptible) UK: Class A US: Schedule II (isomer of Methamphetamine) |
FAQ
1. What is 4-Methylamphetamine (4-MA)?
- 4-Methylamphetamine (4-MA) is a synthetic compound classified as a stimulant and anorectic drug. It falls within the phenethylamine and amphetamine chemical classes.
2. What are the primary effects of 4-MA?
- 4-MA primarily acts as a releasing agent for serotonin, norepinephrine, and dopamine. It is known for its stimulant properties, including increased alertness and energy. However, the specific effects can vary among individuals.
3. Is 4-MA legal?
- The legal status of 4-MA varies from country to country and region to region. Researching and understanding the legality of 4-MA in your specific location is crucial before obtaining or using it.
4. How is 4-MA typically consumed?
- 4-MA is typically consumed orally, but its use can vary. Dosage and administration methods should be cautiously approached due to limited information about its effects and safety.
5. What is its history and research background?
- 4-MA was initially investigated as an appetite suppressant in 1952 and even given the trade name Aptrol. However, its development still needs to be completed. More recently, it has gained attention as a novel designer drug.
6. Are there any safety concerns associated with 4-MA use?
- Yes, there are safety concerns associated with 4-MA use. Limited research means its long-term effects and potential risks must be better understood. Users should exercise extreme caution, adhere to recommended dosages, and prioritize harm reduction practices.
7. Can 4-MA be detected in drug tests?
- Standard drug tests may not typically screen for 4-MA. However, specialized tests may be able to detect its presence if specifically targeted.
8. What should I do if I or someone I know experiences adverse effects from 4-MA use?
- If you or someone you know is experiencing adverse effects from 4-MA use, seek immediate medical attention. Be honest with healthcare professionals about substance use to ensure appropriate care and support.
9. What is the risk associated with the consumption of 4-MA-contaminated substances?
- 4-Methylamphetamine has been associated with contamination in amphetamine (‘speed’) in Europe, leading to several deaths. It highlights the importance of ensuring the purity and safety of substances, as contamination can pose severe health risks.
References
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL conducted a study in May 2005, exploring the “Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs.” Published in “The Journal of Pharmacology and Experimental Therapeutics,” this research aimed to understand the interplay between serotonergic activity and the rewarding properties of amphetamine analogs. (DOI: 10.1124/jpet.104.080101, PMID 15677348, S2CID 12135483)
- In June 2010, Di Giovanni G, Esposito E, and Di Matteo V delved into the “Role of serotonin in central dopamine dysfunction.” This study, published in “CNS Neuroscience & Therapeutics,” explored the intricate relationship between serotonin and dopamine in central nervous system function. (DOI: 10.1111/j.1755-5949.2010.00135.x, PMC 6493878, PMID 20557570)
- Gelvin EP and McGAVACK TH, in January 1952, investigated “2-Amino-1-(p-methylphenyl)-propane (aptrol) as an anorexigenic agent in weight reduction.” Their research, published in the “New York State Journal of Medicine,” explored the potential of aptrol as an appetite suppressant in weight management. (PMID 14890975)
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL revisited the “Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs” in May 2005. This study, published in “The Journal of Pharmacology and Experimental Therapeutics,” further examined the connections between serotonergic activity and the rewarding effects of amphetamine analogs. (DOI: 10.1124/jpet.104.080101, PMID 15677348, S2CID 12135483)
- Baumann MH, Clark RD, Woolverton WL, Wee S, Blough BE, Rothman RB explored “In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat” in April 2011. Published in “The Journal of Pharmacology and Experimental Therapeutics,” this research provided insights into the impact of amphetamine analogs on dopamine transmission. (DOI: 10.1124/jpet.110.176271, PMC 3063744, PMID 21228061)
- In September 2013, Blanckaert P, van Amsterdam J, Brunt T, van den Berg J, Van Durme F, Maudens K, and van Bussel J published a study titled “4-Methyl-amphetamine: a health threat for recreational amphetamine users” in the “Journal of Psychopharmacology.” This research discussed the potential health risks associated with 4-Methylamphetamine, particularly for recreational users of amphetamines. (DOI: 10.1177/0269881113487950, PMID 23784740, S2CID 35436194)