The market situation for 4-Methylthioamphetamine (4-MTA) is characterized by its status as a designer drug and research chemical. 4-MTA is not widely available for sale from traditional sellers or vendors due to its legal restrictions and potential health risks. However, it has occasionally appeared in the market through online channels.
- Limited Availability: 4-MTA is not a mainstream recreational drug and is not typically found for sale on the streets or through traditional drug dealers. Its limited availability is partly due to its legal status in many countries, where it may be classified as a controlled substance.
- Online Presence: Some online vendors specializing in research chemicals may offer 4-MTA for purchase. These vendors often market it as a research chemical for scientific or laboratory purposes. However, the legality of buying and selling 4-MTA can vary by jurisdiction, and consumers should be cautious about potential legal consequences.
- Designer Drug: 4-MTA is a designer drug because it is chemically similar to amphetamines and empathogens. While not as well-documented as other substances, its psychoactive effects are of interest to researchers and may attract individuals seeking novel experiences.
- Regulatory Scrutiny: Due to concerns about its safety and potential for misuse, regulatory authorities closely monitor the sale and distribution of 4-MTA. This can result in periodic legal restrictions or bans in various regions.
In summary, while 4-MTA may be available online from select research chemical vendors, its limited availability and legal restrictions make it a niche product in designer drugs and research chemicals. Individuals interested in obtaining or using 4-MTA should be aware of its consumption’s legal implications and potential health risks.
Contents
- 1 Summary
- 2 History
- 3 Effects
- 4 Metabolism
- 5 FAQ
- 5.1 1. What is 4-Methylthioamphetamine (4-MTA)?
- 5.2 2. How was 4-MTA developed?
- 5.3 3. What are the effects of 4-MTA?
- 5.4 4. Is 4-MTA safe for human consumption?
- 5.5 5. What is serotonin syndrome?
- 5.6 6. Is 4-MTA legal?
- 5.7 7. Can 4-MTA be used recreationally?
- 5.8 8. What should I do if I suspect someone has consumed 4-MTA?
- 5.9 9. Is there ongoing research on 4-MTA?
- 5.10 10. Is there a safe use or dosage for 4-MTA?
- 6 References
Summary
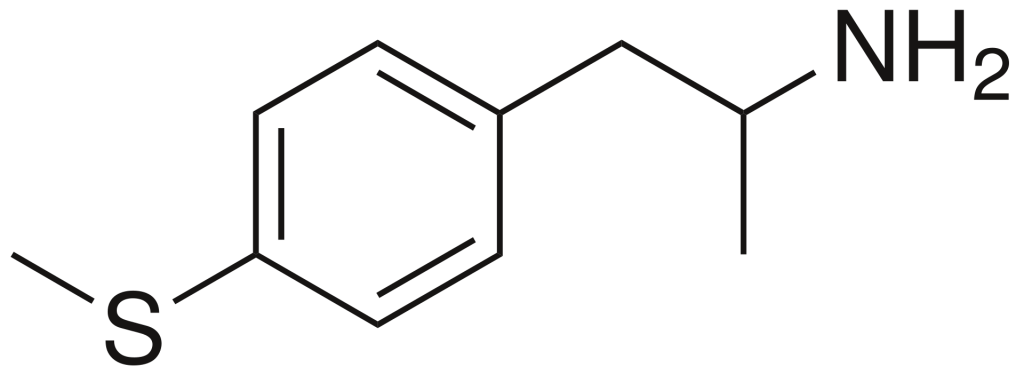
4-Methylthioamphetamine (4-MTA) is a designer drug from the substituted amphetamine class. It was developed during the 1990s by a research team led by David E. Nichols, an accomplished American pharmacologist and medical chemist at Purdue University. In animal studies, 4-MTA has demonstrated its properties as a highly selective serotonin-releasing agent (SSRA) without exhibiting neurotoxic effects. This compound is essentially the methylthio derivative of amphetamine.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 14116-06-4 (R/S) 1030831-15-2 (R) 943816-61-3 (S) |
|---|---|
| PubChem CID | 151900 |
| ChemSpider | 133883 |
| UNII | 6JP2T8KXTR |
| ChEMBL | ChEMBL6467 |
| CompTox Dashboard (EPA) | DTXSID90894854 |
| Chemical and physical data | |
| Formula | C10H15NS |
| Molar mass | 181.30 g·mol−1 |
History
In 1997, the Netherlands Forensic Science Laboratory received alarming reports of three unrelated drug-related deaths. The substance involved was a novel ring-substituted amphetamine derivative. In 1998, two more cases involving this mysterious compound surfaced, and these incidents were brought to the attention of the IPSC (Institut de Police Scientifique et de Criminologie) at the University of Lausanne in Switzerland. Interestingly, this unidentified substance appeared in the hydrochloride salt form in both the Netherlands and Switzerland. Through photo comparisons of different tablets, it became apparent that this compound had also surfaced in other European countries, including the United Kingdom, Germany, and even as far away as Australia. After thorough analytical research, this compound was identified as 4-methylthioamphetamine (4-MTA), originally intended solely for animal pharmacological studies. David Nichols’ studies on 4-MTA were linked to the tablets found across these various countries.
Development: 4-MTA was developed by a research team led by David Nichols [2] to be used exclusively as a laboratory research agent to study the serotonin transporter protein. Nichols was disheartened to discover 4-MTA emerging as a street drug of abuse. He was shocked at realizing that his research had inadvertently contributed to creating a dangerous serotonin-releasing substance, stating, “I was stunned. I had published information that ultimately led to human death.” Nichols initially aimed to comprehend how MDMA functioned in the brain to find a potential therapeutic use for psychotherapy. In this pursuit, Nichols and his team examined molecules with similar structures, including 4-MTA. Between 1992 and 1997, they published three papers detailing the effects of this drug in rats, proposing that it could potentially treat depression and serve as a Prozac alternative. Unbeknownst to Nichols and his team, others synthesized the drug into tablets, known as ‘flatliners’ on the street. Nichols’ laboratory had reported that rats perceived the effects of 4-MTA as similar to ecstasy, likely motivating its production and distribution among humans. Nichols also expressed, “I have never considered my research to be dangerous, and hoped one day to develop medicines to help people.” Following the 4-MTA-related deaths, Nichols’ laboratory was asked to study the human effects of other substances they had researched, aiming to prevent similar situations as with 4-MTA. Most of the molecules previously studied by the laboratory did not pose fatal risks at reasonable dosages.
Use and Availability: Typical street-sold tablets contained approximately 100–140 mg of 4-MTA. Initially, 4-MTA was briefly available in intelligent shops in the Netherlands but was swiftly prohibited by the Dutch government due to severe side effects that began to emerge. The Union of Smartshop Owners removed it from their offerings after discovering that the drug had only been tested on rats. It also briefly appeared on the black market as MDMA during the late 1990s, primarily in the United States. However, it failed to gain popularity due to its high risk of severe side effects, including several reported deaths, and its relatively limited positive euphoric effects.
Effects
4-MTA, akin to paramethoxyamphetamine (PMA), acts as a potent serotonin releaser, leading to marked hyperthermia, potentially resulting in organ failure and even fatality.[better source needed] The primary neuropharmacological impact of 4-MTA is the substantial increase in serotonin release, combined with the inhibition of monoamine oxidase A (MAO-A), which breaks down serotonin. This combination gives rise to a dangerous condition known as serotonin syndrome, characterized by excessive serotonergic activity with potentially fatal consequences. Serotonin syndrome is a hazardous side effect of substances that enhance serotonergic activity. The symptoms of serotonin syndrome induced by 4-MTA are outlined in the Report on the Risk Assessment of 4-MTA:
Symptoms of serotonin syndrome caused by 4-MTA include:
- Euphoria
- Drowsiness
- Sustained rapid eye movement
- Hyperreflexia (exaggerated reflexes)
- Agitation
- Restlessness
- Tachycardia (rapid heart rate)
- Headache
- Clumsiness
- Disorientation
- Intoxication (feeling drunk and dizzy)
- Rigidity
- Rapid muscle contractions and relaxations in the ankle, resulting in abnormal foot movements
- Muscle contractions and relaxations in the jaw
- Muscle twitching leading to hyperthermia
- Shivering
- Elevated body temperature
- Sweating
- Altered mental status (including confusion and a ‘happy drunk state’ called hypomania)[15]
Furthermore, 4-MTA has been found to increase the secretion of various hormones, including adrenocorticotropic hormone (ACTH), corticosterone, prolactin, oxytocin, and renin, by stimulating serotonergic neurotransmission.
There is a suggestion that 4-MTA, due to its slow onset of action, may pose a greater risk than other designer drugs. Substance users often take additional doses rapidly, assuming that the initial dose was insufficient, thus elevating the risk of overdose (EMCDDA, 1999).
Our understanding of the effects of 4-MTA remains limited, primarily due to the scarcity of research and experimental data. Only four studies have been conducted, indicating a weak impact on dopamine and noradrenaline. Notably, these studies involved a single dose of 4-MTA, with no available research on the effects of multiple doses of 4-MTA.
Metabolism
The metabolic breakdown of 4-MTA in humans is believed to follow these postulated steps:
- β-Hydroxylation of the side chain, resulting in the formation of 4-hydroxy-4-methylthioamphetamine (step I).
- Subsequent ring hydroxylation leads to a phenolic structure (step II).
- Oxidative deamination results in the formation of an oxo metabolite. This metabolite can undergo further transformation, including:
- Reduction into the corresponding alcohol (step IIIa).
- Degradation of the side chain into 4-methylthiobenzoic acid (step IIIb).
The primary identified metabolite is 4-methylthiobenzoic acid. Interestingly, this compound can trigger a bioactivation process, which is considered toxic, as it significantly increases sensitivity to reductions in ATP content. The biotransformation pathway of 4-MTA bears significant resemblances to the metabolic pathway of the structurally similar compound, 4-methoxyamphetamine.
FAQ
1. What is 4-Methylthioamphetamine (4-MTA)?
- 4-Methylthioamphetamine (4-MTA) is a chemical compound in the substituted amphetamine class. It is known for its stimulant properties and effects on serotonin release.
2. How was 4-MTA developed?
- 4-MTA was initially synthesised as part of laboratory research into the serotonin transporter protein by a team led by David E. Nichols at Purdue University in the 1990s. It was not intended for recreational use.
3. What are the effects of 4-MTA?
- 4-MTA primarily acts as a serotonin-releasing agent (SSRA) and inhibits serotonin uptake by monoamine oxidase A (MAO-A). This can lead to a condition known as serotonin syndrome, characterized by symptoms like euphoria, rapid eye movement, hyperreflexia, agitation, and high body temperature.
4. Is 4-MTA safe for human consumption?
- No, 4-MTA is not safe for human consumption. It can have severe and potentially life-threatening side effects, including hyperthermia and serotonin syndrome.
5. What is serotonin syndrome?
- Serotonin syndrome is a dangerous condition that occurs when there is excessive serotonin in the body. It can lead to various symptoms, including rapid heart rate, confusion, high body temperature, and even death.
6. Is 4-MTA legal?
- The legal status of 4-MTA varies by country. It is classified as an illegal controlled substance in many places due to its potential for harm.
7. Can 4-MTA be used recreationally?
- Using 4-MTA recreationally is strongly discouraged due to its high risk of severe side effects and the potential for overdose. It is not considered a safe or suitable recreational drug.
8. What should I do if I suspect someone has consumed 4-MTA?
- If you suspect someone has ingested 4-MTA and is experiencing symptoms like hyperthermia or serotonin syndrome, seek immediate medical attention. Providing healthcare professionals with all available information about the substance consumed is crucial.
9. Is there ongoing research on 4-MTA?
- Research on 4-MTA is limited due to its potential dangers. Most studies have focused on its effects in animal models. Research is primarily conducted to understand its mechanisms and potential toxicity.
10. Is there a safe use or dosage for 4-MTA?
- No, there is no known safe use or dosage for 4-MTA. It is not intended for human consumption and poses significant health risks.
References
- Anvisa (2023-07-24). “RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” [Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Published in Diário Oficial da União on 2023-07-25. Archived from the original on 2023-08-27. Retrieved on 2023-08-27.
- Huang X, Marona-Lewicka D, Nichols DE (December 1992). “p-methylthioamphetamine is a potent new non-neurotoxic serotonin-releasing agent”. Published in the European Journal of Pharmacology. Volume 229, Issue 1, Pages 31–38. DOI: 10.1016/0014-2999(92)90282-9. PMID 1473561.
- Li Q, Murakami I, Stall S, Levy AD, Brownfield MS, Nichols DE, Van de Kar LD (December 1996). “Neuroendocrine pharmacology of three serotonin releasers: 1-(1,3-benzodioxol-5-yl)-2-(methylamino)butane (MBDB), 5-methoxy-6-methyl-2-aminoindan (MMAi) and p-methylthioamphetamine (MTA)”. Published in The Journal of Pharmacology and Experimental Therapeutics. Volume 279, Issue 3, Pages 1261–1267. PMID 8968349.
- Murphy J, Flynn JJ, Cannon DM, Guiry PJ, McCormack P, Baird AW, et al. (May 2002). “In vitro neuronal and vascular responses to 5-hydroxytryptamine: modulation by 4-methylthioamphetamine, 4-methylthiomethamphetamine and 3,4-methylenedioxymethamphetamine”. Published in the European Journal of Pharmacology. Volume 444, Issues 1–2, Pages 61–67. DOI: 10.1016/S0014-2999(02)01586-8. PMID 12191583.
- Poortman AJ, Lock E (March 1999). “Analytical profile of 4-methylthioamphetamine (4-MTA), a new street drug”. Published in Forensic Science International. Volume 100, Issue 3, Pages 221–233. DOI: 10.1016/S0379-0738(98)00214-X. PMID 10423848.
- Nutt D, King LA, Saulsbury W, Blakemore C (March 2007). “Development of a rational scale to assess the harm of drugs of potential misuse”. Published in the Lancet. Volume 369, Issue 9566, Pages 1047–1053. DOI: 10.1016/s0140-6736(07)60464-4. PMID 17382831.
- Nichols D (January 2011). “Legal highs: the dark side of medicinal chemistry”. Published in Nature. Volume 469, Issue 7328, Page 7. DOI: 10.1038/469007a. PMID 21209630.
- Błachut D, Wojtasiewicz K, Czarnocki Z, Szukalski B (November 2009). “The analytical profile of some 4-methylthioamphetamine (4-MTA) homologues”. Published in Forensic Science International. Volume 192, Issues 1–3, Pages 98–114. DOI: 10.1016/j.forsciint.2009.08.009. PMID 19766415.
- “Report on the Risk Assessment of 4-MTA in the Framework of the Joint Action on New Synthetic Drugs” (PDF). Published on 1999-05-19. Archived (PDF) from the original on 2020-09-18. Retrieved on 2022-08-21 via European Monitoring Centre for Drugs and Drug Addiction.
- “Ecstasy Or MDMA (also Known As Molly)”. Archived from the original on 2022-08-21. Retrieved on 2022-08-21 from www.dea.gov.
- Elliott SP (March 2000). “Fatal poisoning with a new phenylethylamine: 4-methylthioamphetamine (4-MTA)”. Published in the Journal of Analytical Toxicology. Volume 24, Issue 2, Pages 85–89. DOI: 10.1093/jat/24.2.85. PMID 10732944.
- De Letter EA, Coopman VA, Cordonnier JA, Piette MH (2001). “One fatal and seven non-fatal cases of 4-methylthioamphetamine (4-MTA) intoxication: clinico-pathological findings”. Published in the International Journal of Legal Medicine. Volume 114, Issue 6, Pages 352–356. DOI: 10.1007/s004140100204. PMID 11508803.
- Decaestecker T, De Letter E, Clauwaert K, Bouche MP, Lambert W, Van Bocxlaer J, et al. (2001). “Fatal 4-MTA intoxication: development of a liquid chromatographic-tandem mass spectrometric assay for multiple matrices”. Published in the Journal of Analytical Toxicology. Volume 25, Issue 8, Pages 705–710. DOI: 10.1093/jat/25.8.705. PMID 11765028.
- Smets G, Bronselaer K, De Munnynck K, De Feyter K, Van de Voorde W, Sabbe M (August 2005). “Amphetamine toxicity in the emergency department”. Published in the European Journal of Emergency Medicine. Volume 12, Issue 4, Pages 193–197. DOI: 10.1097/00063110-200508000-00010. PMID 16034267.
- “Report on the Risk Assessment of 4-MTA in the Framework of the Joint Action on New Synthetic Drugs” (PDF). Published on 1999-05-19.
- Błachut D, Wojtasiewicz K, Krawczyk K, Maurin J, Szawkało J, Czarnocki Z (March 2012). “Identification and synthesis of by-products found in 4-methylthioamphetamine (4-MTA) produced by the Leuckart method”. Published in Forensic Science International. Volume 216, Issues 1–3, Pages 108–120. DOI: 10.1016/j.forsciint.2011.09.005. PMID 21982394.
- Ewald AH, Peters FT, Weise M, Maurer HH (September 2005). “Studies on the metabolism and toxicological detection of the designer drug 4-methylthioamphetamine (4-MTA) in human urine using gas chromatography-mass spectrometry”. Published in the Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. Volume 824, Issues 1–2, Pages 123–131. DOI: 10.1016/j.jchromb.2005.07.007. PMID 16027051.
- Carmo H, Hengstler JG, de Boer D, Ringel M, Carvalho F, Fernandes E, et al. (February 2004). “Comparative metabolism of the designer drug 4-methylthioamphetamine by hepatocytes from man, monkey, dog, rabbit, rat and mouse”. Published in Naunyn-Schmiedeberg’s Archives of Pharmacology. Volume 369, Issue 2, Pages 198–205. DOI: 10.1007/s00210-003-0850-0. PMID 14676987.