Summary
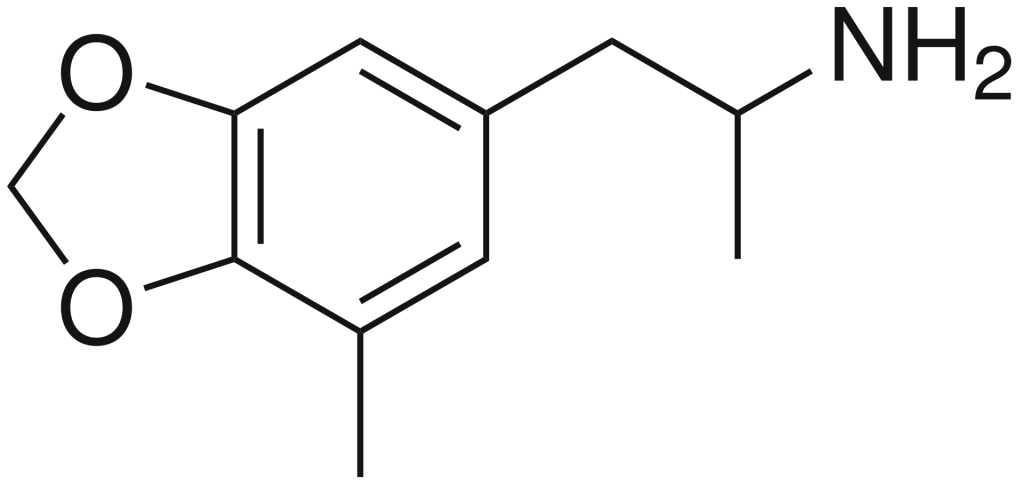
5-Methyl-3,4-methylenedioxyamphetamine (5-Methyl-MDA) is a designer drug belonging to the amphetamine class, known for its entactogenic and psychedelic properties. It is characterized by a methyl group on the amphetamine ring, making it a ring-methylated homolog of MDA and a structural isomer of MDMA.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 749191-14-8 204916-89-2 (HCl) |
|---|---|
| PubChem CID | 10012829 |
| ChemSpider | 8188403 |
| UNII | 10R3PP3FVM |
| ChEMBL | ChEMBL6330 |
| Chemical and physical data | |
| Formula | C11H15NO2 |
| Molar mass | 193.246 g·mol−1 |
Effects and research
Drug discrimination studies have revealed that 5-methyl-MDA can substitute for MDA, MMAI, and LSD but not for amphetamine. This suggests that it elicits entactogenic and hallucinogenic effects while lacking stimulant properties. (Citation needed)
5-Methyl-MDA functions as a selective serotonin-releasing agent (SSRA), with IC50 values of 107nM, 11,600nM, and 1,494nM for serotonin, dopamine, and norepinephrine efflux, respectively. In vitro assays have demonstrated over five times greater potency than MDA. The effective dose in vivo is estimated to be in the range of 15–25 mg. However, subsequent in vivo testing has indicated that it may require at least 100 mg to produce its effects. 2-Methyl-MDA is considerably more potent than MDA but slightly less potent than 5-methyl-MDA. On the other hand, 6-methyl-MDMA (also known as Madam-6) is primarily inactive, possibly due to steric hindrance.
Recent research has leveraged data on 2-methyl-MDA and 5-methyl-MDA to inform computer modeling of the serotonin transporter complex.
Legal status
International Status
5-Methyl-MDA is not classified or scheduled by the United Nations Convention on Psychotropic Substances.
United States:
At the federal level in the United States, 5-Methyl-MDA is not explicitly scheduled. However, it could be regarded as an analog of MDA. In such a case, the sales or possession of 5-Methyl-MDA might be subject to prosecution under the Federal Analogue Act.
FAQ
1. What is 5-Methyl-MDA?
5-Methyl-MDA is a designer drug known for its entactogenic and psychedelic properties. It is structurally related to MDA (3,4-methylenedioxyamphetamine) and MDMA (3,4-methylenedioxymethamphetamine).
2. How does 5-methyl-MDA differ from MDA and MDMA?
5-Methyl-MDA is a ring-methylated homolog of MDA and a structural isomer of MDMA, making it distinct from these substances while sharing some similarities in effects.
3. What are the effects of 5-methyl-MDA?
Studies suggest that 5-methyl-MDA produces entactogenic and hallucinogenic effects without stimulant properties. However, the specific products and experiences can vary among individuals.
4. Is 5-methyl-MDA classified as a controlled substance internationally?
5-Methyl-MDA is not scheduled or classified under the United Nations Convention on Psychotropic Substances.
5. What is the legal status of 5-Methyl-MDA in the United States?
At the federal level in the United States, 5-Methyl-MDA is not explicitly scheduled. However, it might be considered an analog of MDA, potentially subjecting sales or possession to prosecution under the Federal Analogue Act.
6. How potent is 5-Methyl-MDA compared to MDA?
In in vitro assays, 5-Methyl-MDA has demonstrated over five times greater potency than MDA. The effective dose for in vivo effects is estimated to be around 15–25 mg.
7. Is 5-Methyl-MDA widely available or used recreationally?
The availability and use of 5-Methyl-MDA can vary by region and over time. It is essential to stay informed about the legal status and potential risks associated with its use.
8. What research is being conducted on 5-Methyl-MDA?
Recent research has used data on 5-Methyl-MDA and related compounds to inform computer modeling of the serotonin transporter complex, contributing to our understanding of its pharmacological effects.
9. Are there known health risks associated with 5-Methyl-MDA use?
Like many psychoactive substances, 5-Methyl-MDA may carry potential health risks, including hallucinations, paranoia, and other psychological effects. Its long-term effects are not well understood. Users should exercise caution and prioritize harm reduction measures when considering its use.
References
- Parker MA, Marona-Lewicka D, Kurrasch D, Shulgin AT, Nichols DE (March 1998). “Synthesis and pharmacological evaluation of ring-methylated derivatives of 3,4-(methylenedioxy)amphetamine (MDA)”. This research published in the Journal of Medicinal Chemistry delves into the synthesis and pharmacological evaluation of ring-methylated derivatives related to 3,4-(methylenedioxy)amphetamine (MDA). [DOI: 10.1021/jm9705925]
- PIHKAL #98: This reference refers to the entry for 5-Methyl-MDA in “PiHKAL” (Phenethylamines i Have Known And Loved), a comprehensive work by Alexander Shulgin that provides insights into the chemical and pharmacological properties of various compounds.
- Walline CC, Nichols DE, Carroll FI, Barker EL (June 2008). “Comparative molecular field analysis using selectivity fields reveals residues in the third transmembrane helix of the serotonin transporter associated with substrate and antagonist recognition”. Published in The Journal of Pharmacology and Experimental Therapeutics, this study employs comparative molecular field analysis to investigate residues in the third transmembrane helix of the serotonin transporter related to substrate and antagonist recognition. [DOI: 10.1124/jpet.108.136200]
- “Convention on Psychotropic Substances, 1971”. This source contains information about the 1971 Convention on Psychotropic Substances, offering insights into the international scheduling of psychoactive compounds. [Accessed on: 2022-01-19]
- “§1308.11 Schedule I.” This reference pertains to the section of the United States Code of Federal Regulations outlining Schedule I substances. It provides legal information regarding controlled substances in the United States. [Accessed on: 2009-08-27]
- Erowid Analog Law Vault: Federal Controlled Substance Analogue Act Summary. This resource presents a summary of the Federal Controlled Substance Analogue Act as archived in the Erowid Analog Law Vault, providing information on legal regulations concerning analogs of controlled substances.