Dimethocaine, often marketed as a designer drug and sold by various online research chemical sellers, has raised concerns within the scientific and regulatory communities. This synthetic compound, also known as lidocaine, is advertised as a legal alternative to cocaine, making it an attractive prospect for those seeking to buy substances for research purposes. However, it’s crucial to critically evaluate the legitimacy and safety of these vendors and the products they have for sale.
One of the primary concerns with Dimethocaine vendors is the lack of oversight and regulation in the sale of research chemicals. While they may claim that the substance is intended solely for research purposes, the reality is that it can easily find its way into recreational use, potentially putting individuals at risk. The term “designer drug” itself suggests a product created to mimic the effects of controlled substances, raising ethical questions about the intentions behind its sale.
Furthermore, the online availability of Dimethocaine raises concerns about the quality and purity of the product. Research chemicals, by nature, are often unregulated and not subject to the same rigorous testing standards as pharmaceuticals. This lack of oversight can result in variations in purity and potentially dangerous contaminants, posing significant risks to those who purchase these substances.
Another issue is the marketing and advertising strategies employed by these vendors. They often use misleading language to attract buyers, such as claiming that Dimethocaine is a legal high or a safe alternative to illicit drugs. This type of messaging can lead to misconceptions about the substance’s safety and contribute to its misuse.
Contents
- 1 SUMMARY
- 2 History
- 3 Pharmacology
- 4 Metabolism
- 5 side effects
- 6 Toxicity
- 7 Legal status
- 8 FAQ
- 8.1 1. What is Dimethocaine?
- 8.2 2. Is Dimethocaine legal?
- 8.3 3. How does Dimethocaine work?
- 8.4 4. What are the effects of Dimethocaine?
- 8.5 5. Is Dimethocaine safe?
- 8.6 6. Can Dimethocaine be used for research purposes?
- 8.7 7. What are the known risks of Dimethocaine use?
- 8.8 8. What should I do if I suspect someone is abusing Dimethocaine?
- 8.9 9. Can Dimethocaine be detected in drug tests?
- 9 References
SUMMARY
Dimethocaine, also recognized as DMC or larocaine, possesses stimulant properties reminiscent of cocaine, albeit with comparatively reduced potency. Much like cocaine, dimethocaine can induce addiction due to its stimulation of the brain’s reward pathway. Notably, in select countries, dimethocaine is legally marketed as a substitute for cocaine, even earning classification as a “synthetic cocaine derivative” by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Structurally, dimethocaine resembles procaine, characterized by its 4-aminobenzoic acid ester composition, and typically presents as a white powder at room temperature.
However, in a case from June 2010, a product advertised as dimethicone and available for purchase online in the UK was tested, revealing it to be a blend of caffeine and lidocaine. The absence of any dopaminergic stimulant component in such mixtures could account for the limited recreational effects reported by numerous users. In contrast, other analyzed samples have indeed contained authentic dimethocaine. Additionally, a specific “bath salt” product, primarily featuring dimethicone as its active ingredient, garnered attention for its misuse by intravenous drug users in Ireland.
| Identifiers | |
|---|---|
| showIUPAC name | |
| CAS Number | 94-15-5 553-63-9 (HCl) |
| PubChem CID | 7177 |
| ChemSpider | 6909 |
| UNII | R3L4A6GOWZ |
| CompTox Dashboard (EPA) | DTXSID40240185 |
| Chemical and physical data | |
| Formula | C16H26N2O2 |
| Molar mass | 278.396 g·mol−1 |
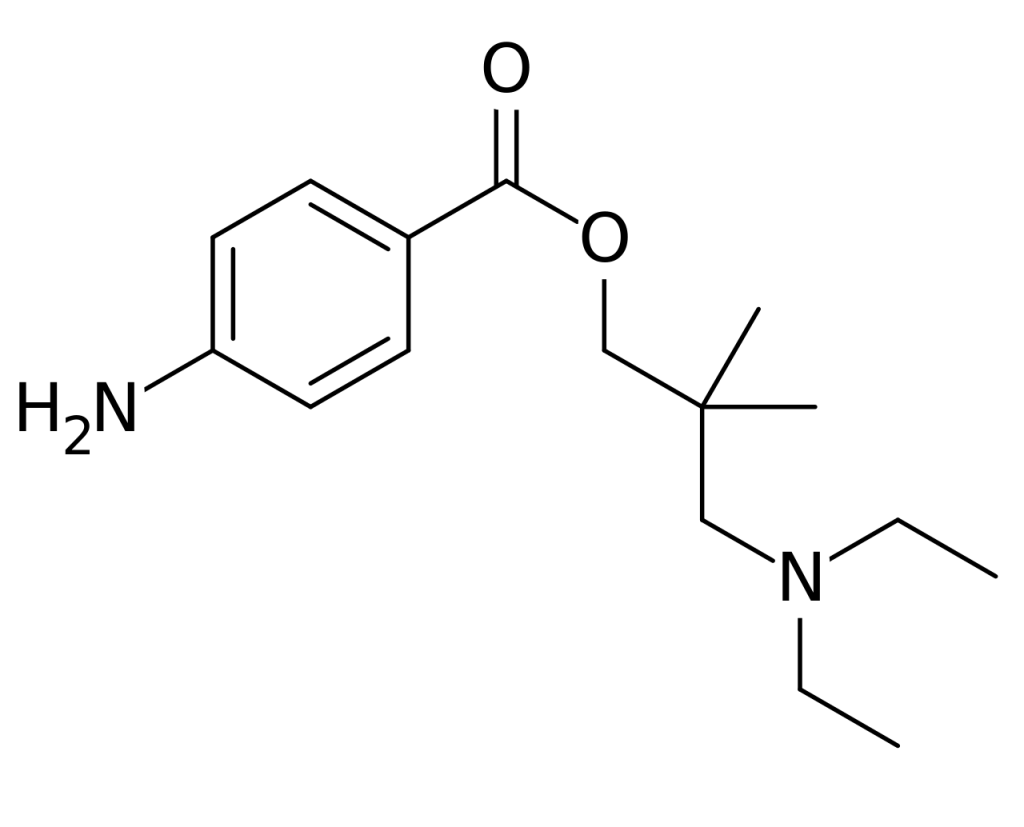
History
Dimethocaine, initially synthesized by the Hoffmann-La Roche company in 1930, found its place in the market under the trade name “larocaine.” During the 1930s, dimethocaine gained favor in the United States, where it was utilized as a local anesthetic. Similar to cocaine and procaine, it played a role in surgical procedures, particularly in fields such as dentistry, ophthalmology, and otolaryngology. However, by the 1940s, it was withdrawn from the market due to its psychoactive effects and the potential for addiction. In contemporary times, dimethicone is unfortunately misused precisely for its psychoactive properties. It is marketed as a substitute for cocaine, often employed to navigate legal restrictions.
Pharmacology
Pharmacodynamics:
Dimethocaine, alongside structurally related local anesthetics like cocaine and procaine, exerts its effects by impeding the reuptake of dopamine (DA) through the inhibition of dopamine transporters (DAT). The dopamine transporter plays a pivotal role in regulating the dynamics of the neurotransmitter dopamine, which in turn governs various functions, including motor control, cognition, and mood. Substances such as cocaine and dimethocaine elicit a euphoric response by hindering dopamine transporters, thereby inducing an overflow of dopamine. Furthermore, dimethocaine has demonstrated the capability to inhibit the binding of CFT, a distinct dopamine uptake inhibitor, in addition to obstructing dopamine uptake. These inhibitory actions underlie the stimulant effects exerted by dimethocaine on the central nervous system.
Both in vivo and in vitro assessments of dopamine transporter activity have substantiated that dimethicone is a potent and efficacious dopaminergic reuptake inhibitor, often referred to as a dopamine indirect agonist. These effects predominantly manifest in the nucleus accumbens, a region situated in the basal forebrain. A comparison of the pharmacological potencies among different local anesthetics has yielded the following order of potency: cocaine > dimethocaine > tetracaine > procaine > chloroprocaine.
Furthermore, the administration of dimethocaine at non-toxic doses has been demonstrated to induce antinociceptive responses in mice. These responses are, at least in part, a consequence of the influence of dimethocaine on the central nervous system. Additionally, a memory-impairing effect observed in mice following dimethicone administration has been proposed to arise from a mechanism of action unrelated to anesthesia.
Pharmacokinetics:
Dimethocaine, when inhaled, commences its action within 10 to 30 minutes, with peak effects occurring between 60 and 120 minutes. The period of active influence extends for 4 to 6 hours, during which “after-effects” persist. These after-effects encompass sensations of fatigue and mild cognitive impairment.

Metabolism
While the precise metabolic pathways of dimethocaine remain uncharted territory, investigations have scrutinized various metabolites in Wistar rats. After the administration of dimethicone, diverse metabolites have been detected and identified within their urine, suggesting the potential existence of distinct metabolic routes. The primary phase I reactions encompass ester hydrolysis, demethylation, hydroxylation of the aromatic system, or a combination of these processes. As for the primary phase II reactions, they encompass N-acetylation, glucuronidation, or a combination of both.
In the initial stages of human metabolism, several cytochrome P450 isozymes play pivotal roles. Specifically, the N-acetylation process is catalyzed by the NAT2 isozyme.
side effects
Much like cocaine, dimethocaine disrupts dopamine uptake in the brain by interfering with dopamine transporters. The potency of these substances hinges on their affinity for dopamine transporters and their ability to hinder dopamine uptake.
Studies conducted with rhesus monkeys have indicated that dimethicone exhibits a lower affinity for dopamine transporters than cocaine. However, its capacity to inhibit dopamine uptake is akin to that of cocaine. Consequently, a higher quantity of dimethocaine is required to elicit a comparable response. The peak effects of this compound manifest within 10 to 20 minutes following administration and return to baseline levels within an hour.
Dimethocaine is frequently misused as a legal alternative to cocaine. It is commonly administered intravenously or nasally, as oral ingestion leads to rapid hydrolysis. The positive effects of dimethocaine encompass euphoria, heightened alertness, increased sociability, and an uplifted mood. However, owing to its cocaine-like actions, it also shares comparable adverse side effects. These detrimental effects encompass tachycardia, breathing difficulties, chest pain, vasoconstriction, insomnia, paranoia, and anxiety. Notably, dimethicone may pose more significant health risks than cocaine due to the necessity for larger doses to achieve the same euphoric sensation, consequently elevating the potential for adverse effects.
Toxicity
Humans:
Cocaine and other local anesthetics are recognized for their potential to induce cardiotoxicity by blocking sodium channels. Nevertheless, there have been no documented reports of dimethocaine exhibiting similar cardiotoxic effects. Regrettably, the research into the toxicity of dimethocaine in humans remains limited, leaving the precise lethal or pharmacological doses undisclosed.
Animals:
In mice, acute toxicity is observed at 40 mg/kg for intravenous administration and 380 mg/kg for subcutaneous injection, which entails injection beneath the skin’s outermost layers. The lethal dose of dimethocaine for mice is 0.3 g per kilogram of body weight.
A study involving mice subjected to an abdominal constriction test entailed subcutaneous administration of dimethocaine at 5, 10, and 20 mg/kg doses. The results revealed dose-dependent antinociceptive responses, which refer to processes inhibiting the sensory neurons’ detection of painful or damaging stimuli.
Furthermore, the elevated plus-maze test unveiled that dimethicone led to impaired memory processes in mice, constituting a toxic effect [Additional information about the test may be included if available].
Legal status
| Legal status | DE: Anlage II (Authorized trade only, not prescriptible) UK: Under Psychoactive Substances Act |
|---|
FAQ
1. What is Dimethocaine?
Dimethocaine, also known as DMC or larocaine, is a synthetic compound with stimulant properties. It has been marketed as a legal alternative to cocaine and has gained popularity in various forms.
2. Is Dimethocaine legal?
The legal status of Dimethocaine varies from country to country. It may be considered legal in some regions for specific purposes like research, but it is essential to check your local laws and regulations before obtaining or using it.
3. How does Dimethocaine work?
Dimethocaine interferes with the reuptake of dopamine in the brain by blocking dopamine transporters. This action results in an increase in dopamine levels, leading to stimulating and euphoric effects.
4. What are the effects of Dimethocaine?
Dimethocaine can induce effects similar to those of cocaine, including euphoria, increased energy, enhanced sociability, and mood elevation. However, it can also produce negative side effects such as tachycardia, difficulty breathing, chest pain, vasoconstriction, insomnia, paranoia, and anxiety.
5. Is Dimethocaine safe?
The safety of Dimethocaine is a subject of concern. Its use can lead to potentially harmful effects, and because it acts similarly to cocaine, it may carry a significant risk of addiction and health complications.
6. Can Dimethocaine be used for research purposes?
Dimethocaine is sometimes used for research purposes, primarily in studies related to its pharmacology and effects on the central nervous system. Researchers should adhere to relevant regulations and ethical guidelines when working with such substances.
7. What are the known risks of Dimethocaine use?
Dimethocaine use can pose risks such as addiction, cardiovascular issues, respiratory problems, and psychological side effects. Due to its limited research and unknown long-term effects, caution is advised.
8. What should I do if I suspect someone is abusing Dimethocaine?
If you suspect someone is abusing Dimethocaine or any other substance, it’s essential to encourage them to seek help from a healthcare professional or addiction specialist. Substance abuse can have serious health consequences, and early intervention is crucial.
9. Can Dimethocaine be detected in drug tests?
Dimethocaine may not be included in standard drug tests. However, specialized tests could detect its presence if specifically screened for.
References
- Meyer MR, Lindauer C, Welter J, Maurer HH (March 2014). “Dimethocaine, a synthetic cocaine analogue: studies on its in-vivo metabolism and its detectability in urine by means of a rat model and liquid chromatography-linear ion-trap (high-resolution) mass spectrometry”. Analytical and Bioanalytical Chemistry. 406 (7): 1845–54. doi:10.1007/s00216-013-7539-0. PMID 24448968. S2CID 10850370.
- Dargan P, Wood D (2013-08-06). Novel Psychoactive Substances: Classification, Pharmacology and Toxicology. Amsterdam: Elsevier/Academic Press. ISBN 978-0-12-415911-2.
- Brandt SD, Sumnall HR, Measham F, Cole J (July 2010). “Second generation mephedrone. The confusing case of NRG-1”. BMJ. 341: c3564. doi:10.1136/bmj.c3564. PMID 20605894. S2CID 20354123.
- An overview of new psychoactive substances and the outlets supplying them Archived November 25, 2011, at the Wayback Machine
- Lindauer C (2014). Toxicokinetics of Emerging Drugs of Abuse: In vivo and in vitro studies on the metabolic fate of the cocaine-derived designer drug dimethocaine. Homburg/Saar: University of Saarland.
- “Dimethocaine – The Drug Classroom”. The Drug Classroom. Retrieved 2018-03-16.
- Woodward JJ, Compton DM, Balster RL, Martin BR (April 1995). “In vitro and in vivo effects of cocaine and selected local anesthetics on the dopamine transporter”. European Journal of Pharmacology. 277 (1): 7–13. doi:10.1016/0014-2999(95)00042-J. PMID 7635175.
- Vaughan RA, Foster JD (September 2013). “Mechanisms of dopamine transporter regulation in normal and disease states”. Trends in Pharmacological Sciences. 34 (9): 489–96. doi:10.1016/j.tips.2013.07.005. PMC 3831354. PMID 23968642.
- Rigon AR, Takahashi RN (June 1996). “The effects of systemic procaine, lidocaine and dimethocaine on nociception in mice”. General Pharmacology. 27 (4): 647–50. doi:10.1016/0306-3623(95)02079-9. PMID 8853299.
- Blatt SL, Takahashi RN (April 1998). “Memory-impairing effects of local anesthetics in an elevated plus-maze test in mice” (PDF). Brazilian Journal of Medical and Biological Research. 31 (4): 555–9. doi:10.1590/s0100-879×1998000400013. PMID 9698809.
- Greene SL (2013). “Miscellaneous Compounds”. Novel Psychoactive Substances. pp. 393–409. doi:10.1016/b978-0-12-415816-0.00017-1. ISBN 9780124158160.
- Meyer MR, Lindauer C, Maurer HH (February 2014). “Dimethocaine, a synthetic cocaine derivative: studies on its in vitro metabolism catalyzed by P450s and NAT2”. Toxicology Letters. 225 (1): 139–46. doi:10.1016/j.toxlet.2013.11.033. PMID 24309420.
- Wilcox KM, Kimmel HL, Lindsey KP, Votaw JR, Goodman MM, Howell LL (December 2005). “In vivo comparison of the reinforcing and dopamine transporter effects of local anesthetics in rhesus monkeys”. Synapse. 58 (4): 220–8. CiteSeerX 10.1.1.327.1264. doi:10.1002/syn.20199. PMID 16206183. S2CID 15631376.
- TOKYO CHEMICAL INDUSTRY CO., LTD. (2012-09-28). “Material Safety Data Sheet Dimethocaine”.
- Mayer LL (1935). “Larocaine, a new anesthetic”. Arch Ophthalmol. 14 (3): 408–411. doi:10.1001/archopht.1935.00840090094004.
- “Tretton ämnen föreslås klassas som narkotika eller hälsofarlig vara” (in Swedish). Folkhälsomyndigheten. 25 September 2019.