The emergence of online vendors offering cathinone, classified as a research chemical, raises critical concerns within the scientific and ethical communities. While these sellers promote cathinones for sale, claiming convenience and accessibility, it is imperative to scrutinize the consequences of this practice.
First and foremost, the credibility and authenticity of cathinone research chemical sellers often come into question. Many vendors operate in a legal gray area, exploiting regulatory loopholes to market and sell substances without rigorous scientific evaluation. This casts doubt on their products’ quality, purity, and safety. Researchers and consumers face the risk of purchasing unreliable compounds, which can compromise experimental outcomes or personal well-being.
Furthermore, the online availability of cathinones perpetuates a problematic atmosphere surrounding research chemicals. The term “research chemical” is often used as a euphemism for substances with psychoactive properties that have not been tested or approved for human use. Such unregulated access to potentially harmful compounds undermines scientific research principles, emphasizing safety, transparency, and ethical conduct.
In addition, the marketing strategies employed by these sellers, including slick websites and enticing promotions, contribute to the normalization of experimental drug use. This normalization can have dire consequences for public health and safety, as it encourages experimentation without a clear understanding of the associated risks.
Moreover, the absence of oversight in the sale of cathinone research chemicals allows these substances to evade legal restrictions. This poses challenges for law enforcement and perpetuates the cycle of designer drugs entering the market as soon as one is banned, making it difficult to control their distribution.
Contents
- 1 Summary
- 2 History
- 3 Legality
- 4 Biological effects
- 5 Chemistry
- 6 FAQ
- 6.1 1. What is Cathinone?
- 6.2 2. How is Cathinone Consumed?
- 6.3 3. What Are the Effects of Cathinone?
- 6.4 4. Is Cathinone Legal?
- 6.5 5. What Are Synthetic Cathinones?
- 6.6 6. What Are the Health Risks Associated with Cathinone Use?
- 6.7 7. How Does Cathinone Affect the Brain?
- 6.8 8. Can Cathinone Be Used Medicinally?
- 6.9 9. Is Cathinone Related to Other Substances?
- 6.10 10. What Is the Biosynthesis of Cathinone?
- 7 References
Summary
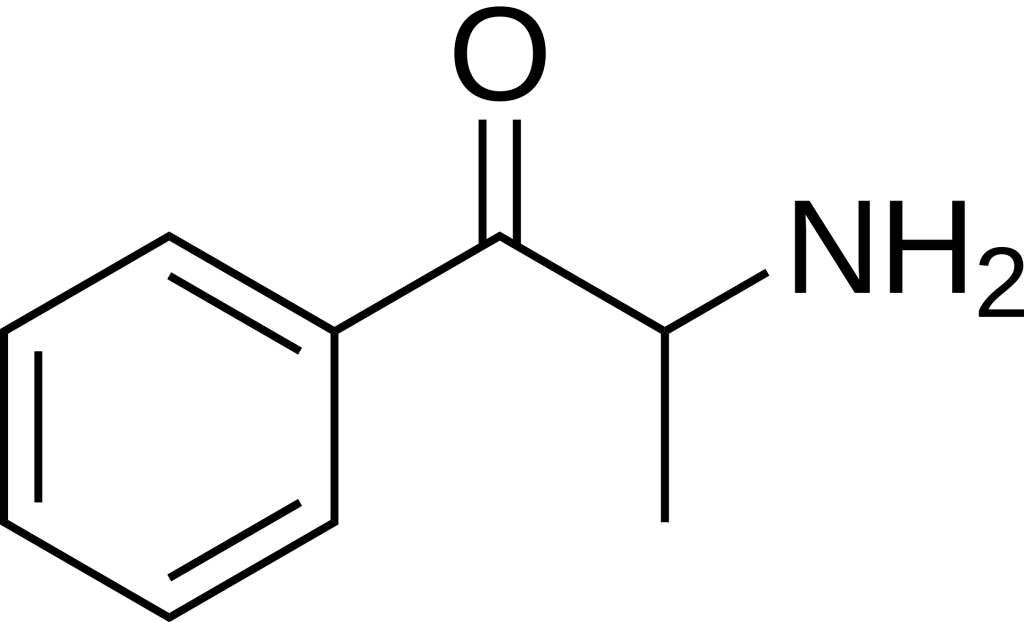
Cathinone, or benzoylethanamine or β-keto-amphetamine, is a monoamine alkaloid primarily sourced from the shrub Catha edulis, commonly known as khat. Chemically akin to ephedrine, cathine, methcathinone, and various other amphetamines, cathinone is believed to be the principal contributor to the stimulating effects associated with Catha edulis, or khat. What sets cathinone apart from numerous other amphetamines is the presence of a ketone functional group in its chemical structure. This structural characteristic is shared with other phenethylamines, including stimulants like methcathinone, MDPV, and mephedrone, as well as the antidepressant bupropion.
| Identifiers | |
|---|---|
| show IUPAC name | |
| CAS Number | 71031-15-7 |
| PubChem CID | 62258 |
| DrugBank | DB01560 |
| ChemSpider | 56062 |
| UNII | 540EI4406J |
| KEGG | C08301 |
| ChEBI | CHEBI:4110 |
| ChEMBL | ChEMBL2104047 |
| CompTox Dashboard (EPA) | DTXSID0050427 |
| ECHA InfoCard | 100.163.927 |
| Chemical and physical data | |
| Formula | C9H11NO |
| Molar mass | 149.193 g·mol−1 |
History
Discovery
Khat, a plant with a long history of cultivation in the Horn of Africa and the Arabian Peninsula, has been used for thousands of years, primarily for the euphoric effects it induces when chewed. Identifying its active components has been a subject of scientific exploration over the years.
In 1930, the active ingredient was first suggested to be cathine, a predominant alkaloid found in the plant. Cathine was believed to be the primary active compound in khat for several decades. However, by the 1960s, it became evident that the amount of cathine in khat leaves needed to be increased to account for the observed effects.
In 1975, a pivotal discovery was made when the United Nations Narcotic Laboratory analyzed khat leaves from Yemen, Kenya, and Madagascar. This analysis revealed the presence of a different alkaloid known as cathinone. While structurally similar to cathine, cathinone was much more abundant in younger khat plants. This revelation led scientists to speculate whether cathinone was the active ingredient responsible for the effects of khat.
In 1994, a study was conducted to investigate the effects of cathinone. Six participants who had never chewed khat were given an active khat sample containing cathinone and a cathinone-free placebo. The study analyzed their moods, activity levels, and blood pressure before and after consumption. The results demonstrated that cathinone produced amphetamine-like effects, confirming that cathinone, not cathine, is the active component responsible for khat’s psychoactive properties.
This discovery shed light on the pharmacological underpinnings of khat’s effects and deepened our understanding of the plant’s active compounds, particularly the role of cathinone.
Cultural Significance
Khat holds immense cultural and economic significance in the Arabian Peninsula and East Africa, with over 20 million people chewing khat leaves daily. It plays a central role in social gatherings, especially in Ethiopia, Kenya, Djibouti, Somalia, and Yemen. Men often chew khat during these gatherings, accompanied by smoking cigarettes and drinking tea. Additionally, it is used by farmers and laborers in the afternoon to combat fatigue and hunger, functioning similarly to caffeine in coffee as an anti-fatigue stimulant. Students and drivers have also been known to use it to stay alert for extended periods.
Freshness is crucial for khat’s desired effects, as fresh leaves have a higher concentration of cathinone. Delaying consumption allows cathinone to break down into the less potent cathine. Historically, khat chewing was prevalent only in regions where the plant grew due to its perishable nature. However, improved transportation methods have facilitated the global spread of khat chewing.
Khat cultivation in Yemen, in particular, has become a highly profitable industry for farmers. Khat plants exhibit varying growth patterns depending on the climate and produce differing amounts of cathinone. Coastal, hot climates are generally conducive to its cultivation, with different regions known for distinct khat varieties, such as the Nehmi khat plant, which boasts the highest concentration of cathinone at 342.5 mg/100g.

Legality
Cathinone is internationally classified as a Schedule I drug under the Convention on Psychotropic Substances. In approximately 1993, the U.S. Drug Enforcement Administration (DEA) included cathinone in Schedule I of the Controlled Substances Act.
Legal Status of Khat The legal status of khat varies worldwide. It is considered legal in some jurisdictions while being prohibited in others (for detailed regulations, refer to Khat (Regulation)). Additionally, substituted cathinones have often served as the primary component in recreational drug blends colloquially known as “bath salts” in the United States.
The following table outlines the legal status of khat and cathinone in different countries:
| Regulation | |
|---|---|
| Eritrea | Legal |
| Ethiopia | Legal |
| Somalia | Legal |
| Djibouti | Legal |
| Kenya | Khat is legal but cathinone and cathine are classified as Class C substances |
| South Africa | Khat is a protected plant |
| China | Illegal |
| Israel | Legal – The khat plant leaves are allowed to be chewed and beverages containing khat are legal, but it is illegal to sell pills based on cathinone extracts |
| Malaysia | Illegal |
| Saudi Arabia | Illegal |
| Yemen | Khat is legal but the cultivation and selling of the plant is regulated by the government |
| Denmark | Illegal |
| Finland | Illegal |
| France | Khat is prohibited as a stimulant |
| Germany | Khat is illegal but a derivative of cathinone is available upon prescription |
| Ireland | Illegal unless authorized |
| Netherlands | Cathinone and cathine have been illegal but khat was announced as illegal in 2012 |
| Norway | Illegal |
| Poland | Illegal |
| Sweden | Illegal |
| Switzerland | Illegal |
| United Kingdom | Illegal |
| Canada | Illegal to obtain unless approved by a medical practitioner |
| United States | Illegal |
| Australia | Khat is regulated under the Australian Customs Service and a special permit is needed to import it for personal use |
| New Zealand | Illegal |
| Georgia | The khat plant itself is allowed to be sold and chewed, but it is illegal to sell or make beverages containing khat |
| Bulgaria | Illegal under List I – “Plants and substances with a high risk to the public health due to their harmful effect of misuse, prohibited for use in human and veterinary medicine” |
Biological effects
Mechanism of Action
Cathinone influences various neurotransmitters in the central nervous system (CNS). It has been observed to stimulate dopamine release while concurrently inhibiting the reuptake of epinephrine, norepinephrine, and serotonin. These neurotransmitters, classified as monoamines, share a common structural feature comprising an aromatic ring and an amine group separated by a two-carbon linker.
Given its hydrophobic nature, cathinone can effectively traverse cell membranes and crucial barriers such as the blood-brain barrier. This property allows cathinone to interact with monoamine transporters within the synaptic cleft between neurons. Consequently, cathinone prompts dopamine release from brain striatal preparations prelabeled with either dopamine or its precursors.
Notably, cathinone’s metabolites, cathine and norephedrine, also possess CNS stimulation properties but elicit considerably weaker effects. The impact of cathinone on the body can be countered by administering a dopamine receptor antagonist, which prevents the synaptic dopamine released by cathinone from binding to dopamine receptors.
Additionally, cathinone can influence cholinergic concentrations in the gastrointestinal tract and airways by blocking prejunctional adrenergic receptors (a2 adrenergic) and activating 5-HT7 receptors, inhibiting smooth muscle contraction. This can lead to side effects such as dry mouth, blurred vision, increased blood pressure, and elevated heart rate.
Pharmacology
Khat leaves, when chewed, release juices containing the alkaloid cathinone. The absorption of cathinone occurs in two phases: one in the buccal mucosa (the lining of the mouth) and the other in the stomach and small intestine. The latter plays a significant role in the absorption of ingested alkaloids. Approximately 2.3 hours after chewing khat leaves, the highest concentration of cathinone in blood plasma is reached, with a mean residence time of 5.2 ± 3.4 hours. Cathinone’s elimination half-life is approximately 1.5 ± 0.8 hours. A two-compartment model for absorption and elimination best describes these pharmacokinetic characteristics. However, only a tiny fraction, up to 7%, of ingested cathinone is recovered in the urine, suggesting significant metabolism within the body. Cathinone is known to metabolize selectively into R, S-(-)-norephedrine and cathine, catalyzed by liver enzymes. The spontaneous degradation of cathinone underscores the necessity of chewing it fresh after cultivation.
Health Effects
Historically, khat has been employed for its medicinal properties since the 10th century, with documented use as an antidepressant due to its ability to induce happiness and excitement. However, chronic khat chewing can lead to drug dependence, as demonstrated in animal studies where subjects increased drug-seeking behavior as dependence developed. The effects of cathinone on individuals can vary, but there is a general behavioral pattern following ingestion:
- Feelings of euphoria lasting one to two hours
- Engaging in severe discussions and heightened irritability
- Heightened imagination
- Entering a depressive stage characterized by irritability, loss of appetite, and insomnia
Beyond CNS-related effects, khat chewing can lead to constipation, heartburn, gum disease, oral cancer, cardiovascular issues, and depression in the long term. Withdrawal symptoms from cathinone use include hot flashes, lethargy, and a strong urge to use the drug, especially during withdrawal.
Chemistry
Biosynthesis
The biosynthesis of S-Cathinone in the khat plant involves a non-beta oxidation pathway, which starts with L-phenylalanine. The process unfolds as follows:
- L-Phenylalanine Conversion:
- The initial step is catalyzed by L-phenylalanine ammonia lyase (PAL). PAL cleaves an ammonia group and forms a carbon-carbon double bond, resulting in cinnamic acid.
- Diverging Pathways:
- At this point, the molecule can follow either a beta-oxidative pathway, yielding benzoyl-CoA, or a non-beta-oxidative pathway, leading to benzoic acid.
- Condensation Reaction:
- A condensation reaction occurs in both pathways, facilitated by a ThDP-dependent enzyme. This enzyme uses pyruvate and produces CO2, converting benzoyl-CoA and benzoic acid into 1-phenylpropane-1,2-dione.
- Transaminase Reaction:
- Subsequently, 1-phenylpropane-1,2-dione undergoes a transaminase reaction, replacing a ketone group with an ammonia group, ultimately forming (S)-cathinone. Notably, (S)-Cathinone can further undergo a reduction reaction to produce the structurally similar but less potent compounds, cathine or norephedrine, also present in the plant.
Besides the beta and non-beta oxidative pathways, cathinone biosynthesis can also involve a CoA-dependent pathway. This pathway combines aspects of both the beta-oxidative and non-beta-oxidative pathways. Initially, it resembles the beta-oxidative pathway, where trans-cinnamic acid from L-phenylalanine is joined to Coenzyme A (CoA). Subsequently, hydration occurs at the double bond. Upon losing CoA, the synthesis continues through the non-beta-oxidative pathway, with benzaldehyde as an intermediate product.
Synthetic Production
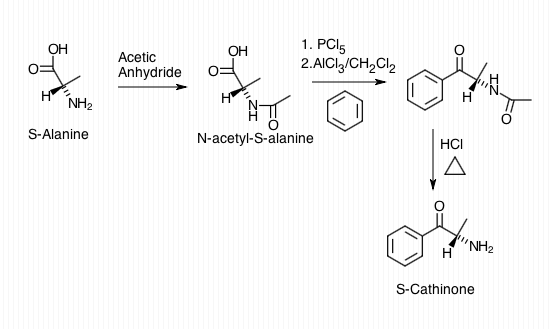
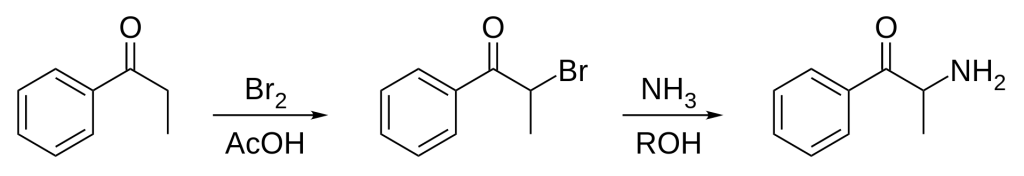
Cathinone can be derived from propiophenone through a Friedel-Crafts acylation of propionic acid and benzene for synthetic production. This process results in propiophenone, which can be brominated, and the bromine can be substituted with ammonia to yield a racemic mixture of cathinone. To produce enantiomerically pure (S)-cathinone, an alternative synthetic approach is employed:
- N-Acetylation:
- Begin with N-acetylation of the optically active amino acid, S-alanine.
- Chlorination:
- Use phosphorus pentachloride (PCl5) to chlorinate the carboxylic acid, forming an acyl chloride. Simultaneously, perform a Friedel-Crafts acylation on benzene with an aluminum chloride catalyst.
- Deacetylation:
- Finally, remove the acetyl-protecting group by heating with hydrochloric acid to produce enantiomerically pure S-(-)-cathinone.
Structure
Cathinone can be found in Catha edulis or synthesized from α-bromopropiophenone, which can be easily obtained from propiophenone. Since cathinone possesses both primary amine and ketone functional groups, it tends to dimerize, particularly when isolated as a free base from plant matter.
The structural likeness of cathinone to other molecules is notable. Reduction of the ketone group leads to cathine, preserving the stereochemistry, or norephedrine if the stereochemistry is reversed. Cathine is a less potent version of cathinone, and the spontaneous reduction of cathinone is why older khat plants are less stimulating than younger ones. Cathinone shares structural similarities with amphetamine, with the sole distinction being the absence of the ketone C=O group. Furthermore, cathinone is structurally akin to methcathinone, much like amphetamine is related to methamphetamine. The primary difference lies in the cathinone’s possession of a ketone oxygen atom (C=O) at the side chain’s β (beta) position.
Several cathinone derivatives exist, featuring adding an R group to the amino end of the molecule. Some of these derivatives have medical applications. For instance, bupropion is a widely prescribed antidepressant with a structure closely related to cathinone, bearing a tertiary butyl group attached to the nitrogen and chlorine affixed to the benzene ring meta- to the primary carbon chain. On the other hand, certain cathinone derivatives serve as potent psychoactive drugs, including methylone, which structurally resembles MDMA.
FAQ
1. What is Cathinone?
Cathinone is a natural stimulant found in the khat plant (Catha edulis) and is chemically related to amphetamines. It is known for its stimulant effects and psychoactive properties.
2. How is Cathinone Consumed?
Cathinone is typically consumed by chewing fresh khat leaves. When chewed, the leaves release the alkaloid cathinone, leading to its stimulant effects. It can also be synthetically produced for various purposes.
3. What Are the Effects of Cathinone?
Cathinone consumption can lead to euphoria, increased alertness, and heightened energy levels. However, it may also result in irritability, loss of appetite, and insomnia during the “crash” phase.
4. Is Cathinone Legal?
The legal status of cathinone varies by country. In some places, such as parts of East Africa, khat leaves containing cathinone are legally chewed as a cultural and social tradition. However, synthetic cathinone, often found in designer drugs, may be illegal in many countries due to its psychoactive and potentially harmful effects.
5. What Are Synthetic Cathinones?
Synthetic cathinones are chemically engineered compounds that mimic the effects of cathinones found in khat. They are often created for recreational drug use and may be sold as “bath salts” or “legal highs.” These synthetic versions can be more potent and dangerous than natural cathinone.
6. What Are the Health Risks Associated with Cathinone Use?
Prolonged or excessive cathinone use can have various health risks, including dependence, cardiovascular issues, gum disease, and oral cancer. It may also lead to depression and other mental health concerns.
7. How Does Cathinone Affect the Brain?
Cathinone affects the central nervous system by stimulating dopamine release and inhibiting the reuptake of epinephrine, norepinephrine, and serotonin. These neurotransmitters play a role in mood, energy, and alertness.
8. Can Cathinone Be Used Medicinally?
While cathinone itself is not typically used for medical purposes due to its psychoactive effects, some derivatives and analogs of cathinone have been investigated for potential medical applications, such as antidepressants.
9. Is Cathinone Related to Other Substances?
Cathinone is structurally related to other substances, including amphetamines, methcathinone, and some antidepressants. These compounds share standard chemical features, but their effects can vary significantly.
10. What Is the Biosynthesis of Cathinone?
Cathinone is biosynthesized in the khat plant through several pathways, including a non-beta oxidation pathway. It starts with L-phenylalanine and involves various enzymatic reactions to produce cathinone.
References
- Anvisa (2023-07-24). “RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” [Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Published in Diário Oficial da União on 2023-07-25. Archived from the original on 2023-08-27. Retrieved on 2023-08-27.
- Toennes SW, Harder S, Schramm M, Niess C, Kauert GF (July 2003). “Pharmacokinetics of cathinone, cathine and norephedrine after the chewing of khat leaves.” Published in the British Journal of Clinical Pharmacology. Volume 56, Issue 1, Pages 125–130. doi:10.1046/j.1365-2125.2003.01834.x. PMC 1884326. PMID 12848785.
- Patel NB (June 2000). “Mechanism of action of cathinone: the active ingredient of khat (Catha edulis).” Published in the East African Medical Journal. Volume 77, Issue 6, Pages 329–332. doi:10.4314/eamj.v77i6.46651. PMID 12858935.
- Widler P, Mathys K, Brenneisen R, Kalix P, Fisch HU (May 1994). “Pharmacodynamics and pharmacokinetics of khat: a controlled study.” Published in Clinical Pharmacology and Therapeutics. Volume 55, Issue 5, Pages 556–562. doi:10.1038/clpt.1994.69. PMID 7910126. S2CID 25788465.
- Kirby A (7 April 2007). “Yemen’s khat habit soaks up water.” Published on BBC News. Archived from the original on 12 October 2014. Retrieved on 20 March 2015.
- Al-Motarreb A, Baker K, Broadley KJ (August 2002). “Khat: pharmacological and medical aspects and its social use in Yemen.” Published in Phytotherapy Research. Volume 16, Issue 5, Pages 403–413. doi:10.1002/ptr.1106. PMID 12203257. S2CID 9749292.
- “List of psychotropic substances under international control” (PDF). Published by the International Narcotics Control Board. United Nations. 2003. Archived from the original (PDF) on 2012-08-31.
- “Synthetic Street Drug Camouflaged as Bath Salts Has Dangerous, Bizarre Effects.” Published on PBS NewsHour on 20 September 2012. Archived from the original on 29 December 2012. Retrieved on 7 December 2013.
- Urquhart C (4 September 2004). “Drugs and dance as Israelis blot out intifada.” Published in The Guardian. Archived from the original on 8 November 2016. Retrieved on 19 April 2015.
- Chai C (16 April 2015). “What you need to know about flakka, the latest drug causing erratic behaviour.” Published on Globalnews.ca. Archived from the original on 20 April 2015. Retrieved on 19 April 2015.
- Extance A. “The rising tide of ‘legal highs’.” Published in Chemistry World. Retrieved on 3 August 2018.
- “НАРЕДБА за реда за класифициране на растенията и веществата като наркотични” [REGULATION on the procedure for classifying plants and substances as narcotic] (PDF). Published by the Ministry of Health (in Bulgarian). Republic of Bulgaria. Archived (PDF) from the original on 2017-08-27. Retrieved on 2017-08-26.
- Huggins KB (17 July 2014). “Cathinone: History, Synthesis, and Human Applications”. Published on Slideshare. Archived from the original on 4 July 2015. Retrieved on 8 March 2015.
- Kalix P (1981). “Cathinone, an alkaloid from khat leaves with an amphetamine-like releasing effect.” Published in Psychopharmacology. Volume 74, Issue 3, Pages 269–270. doi:10.1007/BF00427108. PMID 6791236. S2CID 20621923.
- “Cathinone”. Published on Drug Bank. Drug Bank. Archived from the original on 23 April 2015. Retrieved on 10 March 2015.
- Hagel JM, Krizevski R, Kilpatrick K, Sitrit Y, Marsolais F, Lewinsohn E, Facchini PJ (October 2011). “Expressed sequence tag analysis of khat (Catha edulis) provides a putative molecular biochemical basis for the biosynthesis of phenylpropylamino alkaloids.” Published in Genetics and Molecular Biology. Volume 34, Issue 4, Pages 640–646. doi:10.1590/S1415-47572011000400017. PMC 3229120. PMID 22215969.
- Shulgin A (7 December 2005). “4-Hydroxy-5-methoxy-N,N-dimethyltryptamine, Psilocybe mushrooms, Psilocin”. Available on Ask Dr. Shulgin Online. Archived from the original on 7 September 2013. Retrieved on 10 September 2013.
- “Synthetic cathinones drug profile.” Published by the European Monitoring Center for Drugs and Drug Addiction. EMCDDA. Archived from the original on 17 March 2015. Retrieved on 8 March 2015.