Contents
- 1 Summary
- 2 Adulterant in nutritional supplements
- 3 FAQ
- 3.1 1. What is N,α-Diethylphenethylamine (N,α-DEPEA)?
- 3.2 2. Is N,α-DEPEA a recreational drug?
- 3.3 3. Why is N,α-DEPEA of interest?
- 3.4 4. What are the potential effects of N,α-DEPEA?
- 3.5 5. Is N,α-DEPEA legal?
- 3.6 6. Is N,α-DEPEA safe for consumption?
- 3.7 7. Can N,α-DEPEA be used in medical treatments?
- 3.8 8. How can I stay safe regarding N,α-DEPEA?
- 4 References
Summary
N,α-Diethylphenethylamine (N,α-DEPEA), also known as 2-ethylamino-1-phenylbutane (EAPB), is a chemical compound closely related to methamphetamine and has gained attention as a designer drug. Initially, it was patented by Knoll Pharma as part of a series of analogs intended for potential pharmaceutical applications. Preclinical studies involving these analogs in animal models indicated cognitive enhancement and increased pain tolerance properties. However, despite these promising findings, this class of compounds was not further developed into a pharmaceutical medication. N,α-DEPEA has not undergone human trials. Still, experts such as Pieter Cohen from Harvard Medical School suggest that its potency is likely to be lower than that of methamphetamine but higher than ephedrine.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 119486-07-6 29805-52-5 (HCl) |
|---|---|
| PubChem CID | 14833603 |
| ChemSpider | 27101303 |
| UNII | 7V4PP58Q6T |
| Chemical and physical data | |
| Formula | C12H19N |
| Molar mass | 177.291 g·mol−1 |
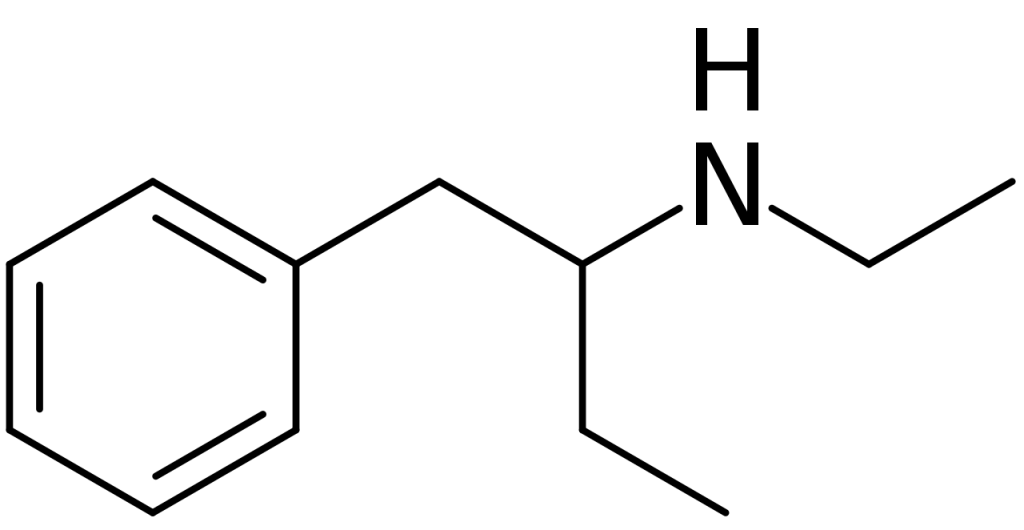
Adulterant in nutritional supplements
In January 2013, South Korean authorities seized the pure substance significantly and anticipated its imminent appearance on the market. Later that same year, in 2013, this substance was discovered as an adulterant in pre-workout supplements, namely Craze (sold by Driven Sports, Inc.) and Detonate (marketed by Gaspari Nutrition). These supplements were deceptively labeled as containing Dendrobium extract.
FAQ
1. What is N,α-Diethylphenethylamine (N,α-DEPEA)?
- N,α-DEPEA is a chemical compound that shares structural similarities with amphetamines and other psychoactive substances. It is considered a substituted phenethylamine.
2. Is N,α-DEPEA a recreational drug?
- N,α-DEPEA is not a widely recognized recreational drug; its psychoactive properties and safety profile are not well-documented. It has gained attention primarily as an adulterant in dietary supplements and pre-workout products.
3. Why is N,α-DEPEA of interest?
- N,α-DEPEA became noteworthy when it was discovered as an adulterant in some dietary supplements, where it was falsely claimed to be a natural extract. This raised concerns about its safety and legality.
4. What are the potential effects of N,α-DEPEA?
- The specific effects of N,α-DEPEA on humans are not well-documented. It is structurally similar to other psychoactive substances, but its pharmacological properties are not widely understood.
5. Is N,α-DEPEA legal?
- The legal status of N,α-DEPEA varies by country and region. In some places, it may be considered a controlled substance or an analog of controlled substances, making its possession, sale, or distribution illegal. It’s essential to check local laws and regulations.
6. Is N,α-DEPEA safe for consumption?
- There is limited scientific research on the safety of N,α-DEPEA in humans. As it is an adulterant in dietary supplements, its security and potential risks are a subject of concern. It’s advisable to avoid products containing undisclosed or unregulated ingredients.
7. Can N,α-DEPEA be used in medical treatments?
- As my knowledge cutoff date in September 2021, N,α-DEPEA had no recognized medical uses. The compound’s potential for therapeutic applications has not been studied extensively.
8. How can I stay safe regarding N,α-DEPEA?
- To ensure safety, it is essential to be cautious about dietary supplements and other products containing undisclosed or unregulated ingredients. Always consult with healthcare professionals and rely on reputable sources of information.
Please note that the knowledge and understanding of N,α-DEPEA may have evolved since my last knowledge update in September 2021. Stay informed about the latest developments and regulations related to this compound.
References
- In November 2015, a study conducted by Wójtowicz M, Jarek A, Chajewska K, Turek-Lepa E, and Kwiatkowska D was published. The study focused on the determination of the designer doping agent, 2-ethylamino-1-phenylbutane, in dietary supplements, and it also included an excretion study following a single oral supplement dose. The research was published in the Journal of Pharmaceutical and Biomedical Analysis, and it can be referenced with the DOI: 10.1016/j.jpba.2015.07.025. The PMID is 26311473.
- In March 2014, Uralets V, App M, Rana S, Morgan S, and Ross W conducted research on designer phenethylamines found in human urine. The study specifically investigated 2-ethylamino-1-phenylbutane and 2-amino-1-phenylbutane. This study was published in the Journal of Analytical Toxicology (Volume 38, Issue 2) and can be found with the DOI: 10.1093/jat/bkt121. The PMID is 24451085.
- Information about “2-Ethylamino-1-phenylbutane” is available from Cayman Chemical and was retrieved on November 4, 2015.
- In 2013, Lee J, Venhuis BJ, Heo S, Choi H, Seol I, and Kim E identified and quantified N,α-diethylphenethylamine in preworkout supplements sold online. This research was published in Forensic Toxicology and can be accessed with the DOI: 10.1007/s11419-013-0205-6. The S2CID is 41372093.
- On October 17, 2013, an article titled “Craze manufacturer disputes NSF’s discovery of drug tainting” was published on Nutraingredients.
- Lee J, Choe S, Choi H, Heo S, Kim E, Kim H, Bang E, and Chung H conducted research on the identification of N-ethyl-α-ethylphenethylamine in crystalline powder seized for suspected drug trafficking. This study was published in Forensic Toxicology in January 2013 and can be referenced with the DOI: 10.1007/s11419-012-0158-1. The S2CID is 13523048.
- An article titled “Popular sports supplements contain meth-like compound” was published in USA Today on October 25, 2013.
- In 2014, Cohen PA, Travis JC, and Venhuis BJ identified a methamphetamine analog, N,α-diethyl-phenylethylamine, in a mainstream dietary supplement. The research was published in Drug Testing and Analysis (Volume 6, Issues 7–8) and can be found with the DOI: 10.1002/dta.1578. The PMID is 24124092, and the S2CID is 42232885.
- A warning was issued over the CRAZE sports supplement in the New Zealand Herald on November 13, 2013.