Contents
- 1 Summary
- 2 History and culture
- 3 Chemistry
- 4 Pharmacology
- 5 Legal status
- 6 FAQ
- 6.1 1. What is N-Ethylhexedrone?
- 6.2 2. Is N-Ethylhexedrone legal?
- 6.3 3. How is N-Ethylhexedrone typically used?
- 6.4 4. What are the effects of N-Ethylhexedrone?
- 6.5 5. What are the potential risks and side effects of N-Ethylhexedrone?
- 6.6 6. Is N-Ethylhexedrone addictive?
- 6.7 7. Can N-Ethylhexedrone be tested for in drug screenings?
- 6.8 8. How should N-Ethylhexedrone be stored?
- 6.9 9. Can I buy N-Ethylhexedrone online?
- 6.10 10. Is N-Ethylhexedrone safe for human consumption?
- 7 References
Summary
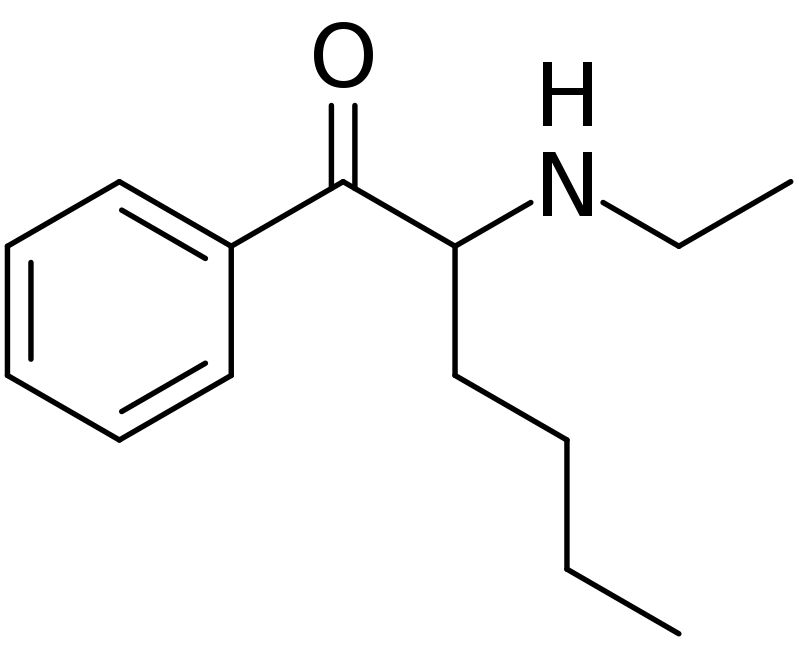
N-ethylhexedrone, also known as α-ethylaminocaprophenone, Hexen, NEH, and N-ethylnorhexedrone, belongs to the cathinone class of stimulants. It functions as a norepinephrine–dopamine reuptake inhibitor (NDRI), exhibiting IC50 values of 0.0978 μM for norepinephrine and 0.0467 μM for dopamine, respectively.
The origins of N-Ethylhexedrone trace back to the 1960s when it was initially documented in a series of patents by Boehringer Ingelheim. This research eventually led to the development of the more well-known drug, methylenedioxypyrovalerone (MDPV), although this information may require verification.
Starting in the mid-2010s, N-ethylhexedrone has been readily available for purchase online, marketed as a designer drug. In 2018, it gained notoriety as the second most frequently identified drug from the cathinone class in seizures by the Drug Enforcement Administration (DEA).
The compound itself was first synthesized by Boehringer Ingelheim in 1964 and subsequently appeared on the online research chemical market around late 2015. It falls into the category of novel psychoactive substances strategically designed to mimic the effects of prohibited substances, allowing them to evade drug regulations. These substances are collectively known as “bath salts.”
User reports describe N-ethylhexedrone as producing euphoric stimulant effects akin to those of crack-cocaine and α-PVP-type compounds, particularly when administered through insufflation or vaporization. Similar to other substituted cathinones, N-ethylhexedrone has gained attention due to its association with compulsive redosing and the development of addictive behaviours when misused.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 802857-66-5 HCl : 18410-62-3 |
|---|---|
| PubChem CID | 134822125 |
| ChemSpider | 58838620 |
| UNII | TAX3KSX6GY HCl : BH93PZ33WU |
| Chemical and physical data | |
| Formula | C14H21NO |
| Molar mass | 219.328 g·mol−1 |
History and culture
N-Ethylhexedrone was originally patented in 1964 by the German pharmaceutical company Boehringer Ingelheim, with the aim of exploring its potential as an anorexigenic agent. The patent not only outlines its synthesis but also includes other derivatives of aminoketone.
Remarkably, this substance quickly gained traction in the New Psychoactive Substances (NPS) market across various European countries. Its presence was initially detected in a sample received by the JRC (Joint Research Centre) from the Belgian Customs laboratory in November 2015. Subsequently, in January 2016, it was identified by the JRC in another sample provided by French Customs. Following this, in February 2016, several countries, including Sweden, The Netherlands, France, Belgium, and Slovenia, reported the identification of this substance.
By 2017, N-Ethylhexedrone had become the most frequently seized cathinone in the European Union (EU), Norway, and Turkey. In 2018, it remained a prevalent cathinone, second only to pentylone, among substances identified in seizures by the Drug Enforcement Administration (DEA).
Chemistry
N-Ethylhexedrone is a chemical derivative of hexedrone, featuring the replacement of the methyl group attached to the nitrogen atom with an ethyl group. This structural modification renders it closely related to pentedrone and α-pyrrolidinohexiophenone (A-PHP). The key difference lies in the substitution of a pyrrolidine group with an N-ethyl group.
This compound belongs to the cathinone chemical class, characterized by its molecular structure. The term “substituted cathinone” encompasses a wide range of substances based on cathinone, which is the primary active component found in the khat plant. Cathinone is essentially composed of an amphetamine core, consisting of a phenethylamine core with an alkyl group attached to the alpha carbon and an oxygen group affixed to the beta carbon. Cathinones are commonly referred to as the beta-ketone (βk) analogues of amphetamines, distinguished by a double-bonded oxygen at the β-carbon. Notably, the cathinone framework can be altered in three distinct locations, resulting in numerous potential compounds. These modifications can occur at the aromatic ring (R2-R5), the alpha carbon (Rα), or the amine group (RN1, RN2).
In the case of N-ethylhexedrone, there are two specific substitutions. Firstly, at the Rα position, a n-butyl substitution forms a hexan chain. Secondly, an ethyl group is attached to the amine group at RN2, giving rise to the designation “N-ethyl.”
Pharmacology
There is a limited amount of data available regarding the human pharmacokinetics and pharmacodynamics of N-ethylhexedrone and several other recently introduced substituted cathinones, primarily documented in post-mortem cases of overdose. Similar to amphetamines, synthetic cathinones exert their stimulating and sympathomimetic effects by elevating the synaptic concentrations of key catecholamines such as dopamine, serotonin, and norepinephrine. These compounds function by inhibiting the reuptake transporters responsible for monoamines, resulting in reduced clearance of these neurotransmitters from the synapses. Additionally, they can induce the release of biogenic amines stored within cells. It is worth noting that N-ethylhexedrone exhibits a notable affinity for the dopamine transporter.
Given its structural characteristics and the assumption that N-ethylhexedrone follows a metabolic pathway similar to other cathinones, it is likely that this compound undergoes processes such as N-dealkylation and reduction of the carbonyl group, followed by subsequent N-dealkylation.
Synthetic cathinones generally possess a lower ability than amphetamines to penetrate the blood-brain barrier due to the presence of a beta-keto group, which increases polarity. In contrast, pyrrolidine derivatives, such as some synthetic cathinones, have an enhanced ability to cross the blood-brain barrier, attributed to the low polarity conferred by the pyrrolidine ring. Investigations into the metabolism of synthetic cathinones have revealed common transformations, including N-demethylation, reduction of the keto group to a hydroxyl group, and oxidation of ring alkyl groups.

Legal status
N-Ethylhexedrone gained international recognition when it was incorporated into the UN Convention on Psychotropic Substances as a Schedule II controlled substance in March 2020. Here is its legal status in various countries:
- Brazil: Since June 5, 2017, N-ethylhexedrone has been illegal to possess, produce, or sell in Brazil, listed under Portaria SVS/MS nº 344.
- Canada: N-Ethylhexedrone is classified as a Schedule I controlled substance in Canada.
- Germany: As of November 26, 2016, N-Ethylhexedrone is subject to control under the NpSG (New Psychoactive Substances Act) in Germany. Activities such as production, import for market placement, administration to others, marketing, and trading are punishable offences. While possession is illegal, it is not punishable. There is a possibility that ordering N-ethylhexedrone could be considered an incitement to place it on the market according to legislation.
- Hungary: Hungary controls N-Ethylhexedrone as a new psychoactive substance.
- Ireland: N-Ethylhexedrone is controlled under SI 173/2017 in Ireland, listed under Schedule 1, paragraph 1(b), as it is structurally derived from 2-amino-1-phenyl-1-propanone and possesses an ethyl group in the 3-position of the propanone side-chain.
- Japan: In Japan, N-ethylhexedrone is classified as a controlled substance.
- Sweden: N-Ethylhexedrone was identified as a potentially dangerous substance in Sweden on June 21, 2016. While it is controlled, it is neither classified as a narcotic nor completely outlawed.
- Switzerland: In Switzerland, N-Ethylhexedrone can be considered a controlled substance under Verzeichnis E point 1 as a defined derivative of Cathinone. However, it is legal for scientific or industrial use.
- United Kingdom: N-Ethylhexedrone falls under Class B drug classification in the United Kingdom due to the cathinone catch-all clause.
- United States: The DEA (Drug Enforcement Administration) placed N-Ethylhexedrone in Schedule I through a temporary scheduling order in July 2019, and it became permanently classified as Schedule I in June 2022.
FAQ
1. What is N-Ethylhexedrone?
N-ethylhexedrone, also known as Hexen or NEH, is a synthetic stimulant compound belonging to the cathinone class. It is chemically related to other cathinones like alpha-PVP and MDPV. N-ethylhexedrone is known for its stimulant effects and has gained popularity as a research chemical.
2. Is N-Ethylhexedrone legal?
The legal status of N-Ethylhexedrone varies by country and region. It is often considered a controlled substance in many places due to its psychoactive properties. It is crucial to research and understand the legal status of N-Ethylhexedrone in your area before attempting to purchase or possess it.
3. How is N-Ethylhexedrone typically used?
N-ethylhexedrone is most commonly used for research purposes and is not approved for human consumption. Researchers may acquire it for scientific investigations and pharmacological studies. Its effects on the central nervous system are similar to other stimulants, but its safety profile is not well-documented.
4. What are the effects of N-Ethylhexedrone?
N-ethylhexedrone is reported to produce stimulating and euphoric effects. Users have described increased energy, alertness, and sociability. However, it can also lead to side effects such as anxiety, paranoia, and increased heart rate. The effects and risks can vary depending on the dose and individual tolerance.
5. What are the potential risks and side effects of N-Ethylhexedrone?
N-Ethylhexedrone has a limited history of human use, and its safety profile is not well-established. Potential risks and side effects may include anxiety, paranoia, increased blood pressure, heart palpitations, insomnia, and even addiction. Long-term effects are not well understood.
6. Is N-Ethylhexedrone addictive?
Like many stimulant substances, N-Ethylhexedrone has the potential to be habit-forming and lead to addiction, especially with frequent or high-dose use. It is important to exercise caution and use it responsibly if used for research purposes.
7. Can N-Ethylhexedrone be tested for in drug screenings?
N-ethylhexedrone is not typically included in standard drug tests. However, specialized tests that screen for synthetic cathinone may detect its presence if administered.
8. How should N-Ethylhexedrone be stored?
If you are a researcher and working with N-Ethylhexedrone, it should be stored in a secure and controlled environment. It should be kept away from heat, light, and moisture to maintain its stability.
9. Can I buy N-Ethylhexedrone online?
The availability of N-Ethylhexedrone for purchase online may vary depending on your location and local laws. However, it’s important to note that purchasing and using this compound may have legal consequences in many jurisdictions. Always research and comply with local regulations.
10. Is N-Ethylhexedrone safe for human consumption?
No, N-Ethylhexedrone is not considered safe for human consumption, and its effects on humans are not well-documented. It is primarily used for research purposes, and the potential risks associated with its consumption are significant. It is essential to prioritize safety and adhere to legal guidelines when dealing with this substance.
References
- Anvisa Regulation (RDC Nº 804): The Brazilian Health Regulatory Agency (Anvisa) issued Regulation No. 804, which pertains to Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control. This regulation is aimed at controlling certain substances with psychoactive properties.
- Chemical Characterization: Studies, such as one conducted by Matsuta et al. in 2014, have focused on the structural characterization of cathinone-type designer drugs, including N-Ethylhexedrone, using mass spectrometry.
- Crystal Structure Analysis: Research conducted by Kuś et al. in July 2019 involved the spectroscopic characterization and crystal structure analysis of several hydrochloride cathinones, including N-Ethylhexedrone.
- Biogenic Amine Transporters: A study by Eshleman et al. in March 2019 explored the structure-activity relationships of synthetic cathinones and benzofurans concerning their effects on biogenic amine transporters.
- Synthetic Cathinones History: The synthesis of cathinone dates back several decades, as evidenced by a patent from 1965 (DE 1545591) describing the production of α-Aminoketones with a heterocyclic amino group, a class of compounds that includes cathinone.
- Abuse Potential: Research by Kolanos et al. in December 2013 examined the “deconstruction” of the synthetic cathinone MDPV and its effects on the human dopamine transporter, shedding light on the abuse potential of such compounds.
- Analytical Reports: Analytical reports, such as the one provided in the European Project RESPONSE, have been instrumental in identifying and characterizing synthetic cathinones like N-Ethylhexedrone.
- Identification of Derivatives: Liu et al., in August 2017, conducted research to identify and analytically characterize nine synthetic cathinone derivatives, including N-Ethylhexedrone, highlighting the need for robust analytical methods.
- DEA’s Monitoring: The Drug Enforcement Administration (DEA) published an “Emerging Threat Report” in 2018, emphasizing the agency’s role in monitoring new psychoactive substances, including N-Ethylhexedrone.
- WHO Recommendations: The World Health Organization (WHO) recommended the scheduling of certain psychoactive substances, including synthetic cathinones, in December 2019, highlighting the global efforts to control these substances.
- Legal Status: Different countries have taken various approaches to regulating synthetic cathinones. For example, Brazil has its regulations (e.g., RDC No. 159), while Germany has implemented the New Psychoactive Substances Act (NpSG).
- Toxicological Studies: Toxicological studies have investigated the effects and potential risks of N-Ethylhexedrone, including cases of fatal intoxication and mixed poisonings involving synthetic cathinone.
- Behavioural Effects: Studies, such as the one by Gatch et al. in July 2021, have explored the behavioural effects of synthetic cathinones in rodents, shedding light on their pharmacological properties.
- Monoamine Transporters Inhibition: Research by Cozzi et al. in September 1999 highlighted the inhibition of plasma membrane monoamine transporters by beta-keto amphetamines, a class that includes synthetic cathinones.
- Metabolism: Meyer and Maurer’s review in June 2010 discussed the metabolism of designer drugs of abuse, providing insights into how synthetic cathinones are metabolized in the body.