Methamphetamine, a potent stimulant with a high potential for abuse and addiction, has long been a concern for public health authorities. In recent years, the emergence of online sellers offering this dangerous substance as a “research chemical” has raised even greater alarm. While the internet has undoubtedly expanded access to legitimate scientific research materials, it has also facilitated the distribution of illicit and harmful substances.
The term “research chemical” is often exploited by unscrupulous vendors who sell methamphetamine online. These sellers cynically market their products as tools for scientific inquiry, thereby skirting legal restrictions. This blatant misrepresentation endangers public health and undermines the credibility of genuine scientific research.
Buying methamphetamine from such online vendors is a reckless and potentially life-threatening choice. The lack of quality control, purity standards, and dosage guidelines associated with these illicit transactions poses significant risks to the buyer. Users have no way of knowing what they are genuinely getting, which can lead to overdoses, severe health complications, or even death.
Moreover, the availability of methamphetamine for sale online perpetuates the devastating impact of addiction on individuals, families, and communities. The ease of access exacerbates the existing epidemic of methamphetamine abuse, causing immeasurable harm.
Authorities and law enforcement agencies must crack down on these rogue sellers who exploit the guise of “research chemicals.” Stricter regulations, rigorous enforcement, and public awareness campaigns are essential to combat this growing problem.
Contents
- 1 Summary
- 2 History and culture
- 3 Chemistry
- 4 Pharmacology
- 5 Subjective effects
- 6 Toxicity
- 7 Legal status
- 8 FAQ
- 8.1 1. What is methamphetamine?
- 8.2 2. How is methamphetamine different from amphetamine?
- 8.3 3. How is methamphetamine used?
- 8.4 4. What are the short-term effects of methamphetamine use?
- 8.5 5. What are the long-term effects of methamphetamine use?
- 8.6 6. Is methamphetamine addictive?
- 8.7 7. Are there any medical uses for methamphetamine?
- 8.8 8. What are the legal consequences of methamphetamine possession and distribution?
- 8.9 9. How can I get help for methamphetamine addiction?
- 8.10 10. What are the dangers of methamphetamine use?
- 8.11 11. Is there any harm reduction advice for methamphetamine users?
- 8.12 12. Can methamphetamine use lead to mental health issues?
- 8.13 13. Is there any research on the medical benefits of methamphetamine?
- 9 References
Summary
N-Methylamphetamine, commonly known as Methamphetamine or by its street names such as Ma, Meth, Glass, Ice, Shard, Crank, Tina, T, Tweak, Yaba, and Crystal, falls within the amphetamine class of classical stimulant substances. While structurally related to amphetamine, Methamphetamine exhibits a unique property by rapidly crossing the blood-brain barrier owing to its relatively high lipid solubility. Its primary mechanism of action involves elevating levels of crucial neurotransmitters in the brain, including serotonin, dopamine, and norepinephrine.
This compound found its origin in the year 1893 when Japanese chemist Nagayoshi Nagai synthesized it from ephedrine. Despite its pharmaceutical beginnings, Methamphetamine, much like heroin and cocaine, has garnered a notorious reputation as a dangerous and highly addictive “street drug.”
Users of Methamphetamine report a range of subjective effects, including heightened motivation, increased stamina, reduced appetite, enhanced libido, and euphoria. However, chronic, high-dose usage can lead to adverse psychological states such as anxiety, paranoia, delusions, thought disorganization, psychosis, and even violent behaviour. Notably, Methamphetamine is often associated with compulsive redosing, mainly when administered through vaporization or injection, due to the euphoric rush it initially induces.
One of the most concerning aspects of Methamphetamine is its exceptionally high potential for abuse and addiction, primarily attributed to the overwhelming euphoria it generates. Unlike therapeutic doses of amphetamine, recreational usage of moderate to high amounts of Methamphetamine is considered directly neurotoxic to humans, causing damage to both dopamine and serotonin neurons within the central nervous system. This neurotoxicity is further corroborated in nonhuman mammals, where degeneration of monoaminergic terminals and neuronal apoptosis have been observed. In humans, the neurotoxic effects persist. Furthermore, Methamphetamine exhibits cardiotoxic properties, elevating blood pressure and increasing the risk of stroke and heart attack.
Given the inherent risks associated with Methamphetamine use, it is strongly recommended that harm reduction practices be rigorously employed when engaging with this substance.
| Identifiers | |
|---|---|
| show IUPAC name | |
| CAS Number | 537-46-2 (dl)-Methamphetamine hydrochloride: 300-42-5 |
| PubChem CID | 1206 |
| IUPHAR/BPS | 4803 |
| DrugBank | DB01577 |
| ChemSpider | 1169 |
| UNII | 44RAL3456C(dl)-Methamphetamine hydrochloride: 24GNZ56D62 |
| KEGG | D08187 |
| ChEBI | CHEBI:6809 |
| ChEMBL | ChEMBL1201201 |
| PDB ligand | B40 (PDBe, RCSB PDB) |
| CompTox Dashboard (EPA) | DTXSID8037128 |
| ECHA InfoCard | 100.007.882 |
| Chemical and physical data | |
| Formula | C10H15N |
| Molar mass | 149.237 g·mol−1 |
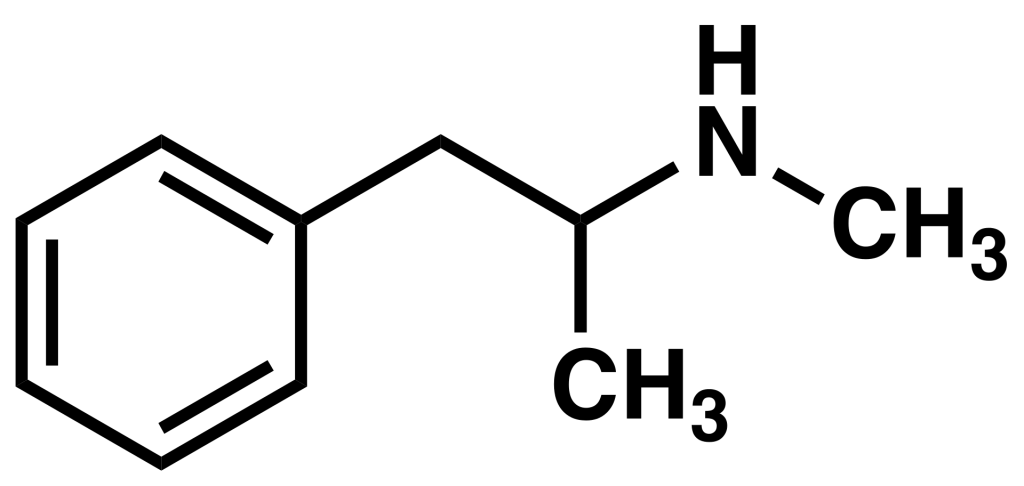
History and culture
Amphetamine’s origins trace back to 1887 when Romanian chemist Lazăr Edeleanu first synthesized it in Germany and christened phenylisopropylamine. In a parallel development, methamphetamine was created by Japanese chemist Nagai Nagayoshi in 1893, derived from ephedrine. However, neither substance found pharmacological utility until 1934 when Smith, Kline, and French introduced amphetamine as an inhaler called Benzedrine, primarily marketed as a decongestant.
The significance of amphetamine and methamphetamine escalated during World War II, as Allied and Axis forces widely employed them for their stimulating and performance-enhancing attributes.
As the addictive nature of these substances became apparent, governments worldwide initiated stringent regulations on their distribution. For instance, in 1970, the United States classified methamphetamine and amphetamine as Schedule II controlled substances under the Controlled Substances Act.
Despite these stringent controls, both amphetamine and methamphetamine have continued to be used legally or illicitly by individuals from diverse backgrounds for various purposes. Due to the expansive underground market for these substances, clandestine chemists often illicitly synthesize them, leading to trafficking and illegal sales on the black market. It’s worth noting that, based on drug and drug precursor seizures, the illicit production and trafficking of methamphetamine appear more prevalent than amphetamine.
Notably, methamphetamine hydrochloride has gained approval from the United States Food and Drug Administration (USFDA) under the “Desoxyn.” However, its prescription remains rare, primarily due to its high potential for abuse. Typically, it is only considered for use in severe cases of obesity or ADHD when all other treatment options have been exhausted.
Chemistry
Methamphetamine, also known as N-methylamphetamine, belongs to the synthetic amphetamine family. These amphetamine class molecules share a standard phenethylamine core structure characterized by a phenyl ring attached to an amino (NH2) group through an ethyl chain, with an additional methyl substitution at the Rα position. Essentially, amphetamines are phenethylamines that are alpha-methylated. Methamphetamine, in particular, includes a further methyl substitution at the RN position, a feature it shares with other substances such as MDMA, methcathinone, and mephedrone.
Methamphetamine exhibits two distinct stereoisomers: dextrorotary and levorotary. Dextrorotatory, often called d-methamphetamine, is a more potent central nervous system (CNS) stimulant than levomethamphetamine. However, both isomers are recognized for their potential to lead to dependence and addiction when misused. At high recreational doses, they can produce similar toxic effects, underscoring the importance of responsible use and awareness of the risks associated with these substances.
Pharmacology
Methamphetamine predominantly influences the central nervous system (CNS) by functioning as a releasing agent for critical neurotransmitters, including dopamine, norepinephrine, and serotonin. Furthermore, it serves as a reuptake inhibitor for select transporter neurons, thereby retaining neurotransmitters like norepinephrine within the synaptic cleft. Methamphetamine also functions as a reverse transporter for specific neurons that transport monoamines. This action enhances monoamine levels by expelling neurotransmitters from their storage vesicles into the synaptic gap, compelling dopamine transporters to operate in reverse.
Other means by which methamphetamine elevates monoamine levels include:
- Reducing the presence of dopamine transporters on the cell surface yields a similar effect as previously described.
- Augmenting cytosolic monoamine levels through inhibiting monoamine oxidase (MAO) activity.
- Enhancing the activity and expression of tyrosine hydroxylase (TH), the enzyme responsible for dopamine synthesis.
Furthermore, owing to its high lipid solubility, methamphetamine readily traverses the blood-brain barrier, resulting in a rapid onset of effects compared to other stimulants. This phenomenon leads to reward, euphoria, and heightened stimulation, coupled with an unfavourable offset.

Subjective effects
Disclaimer: The ensuing effects described below are drawn from the Subjective Effect Index (SEI), a compilation of anecdotal user reports and insights from contributors to PsychonautWiki. Given their origin, these effects should be approached with scepticism.
Additionally, it is essential to recognize that these effects may manifest differently or consistently. However, it is generally observed that higher doses are more likely to produce a broader spectrum of products. It is crucial to remember that adverse effects become increasingly probable with higher doses, potentially encompassing addiction, severe harm, or even fatality.
Physical:
- Stimulation: Methamphetamine is renowned for its pronounced and energetic inspiration, akin to amphetamine but notably more robust than modafinil, caffeine, and MDMA. This stimulation often compels individuals to engage in physical activities such as dancing, socializing, running, or cleaning. Higher doses can lead to involuntary bodily shakes and vibrations, jaw clenching, and an inability to remain still.
- Physical Euphoria: Methamphetamine can induce intense physical euphoria, particularly when vaporized or injected. However, this initial euphoric rush may fade before the substance’s effects wane, potentially promoting compulsive redosing and accruing harmful consequences.
- Abnormal Heartbeat
- Increased Blood Pressure
- Increased Heart Rate
- Appetite Suppression
- Body Odor Alteration: Methamphetamine might impart a distinct odour to bodily secretions like urine and sweat, which some may find unpleasant while others may enjoy.
- Bronchodilation
- Dehydration
- Frequent Urination
- Increased Bodily Temperature
- Increased Perspiration
- Muscle Contractions
- Muscle Spasms
- Neurotoxicity: Especially with prolonged use.
- Stamina Enhancement: More pronounced than with other commonly used stimulants.
- Tactile Enhancement
- Tactile Hallucination: High doses and extended use can lead to hallucinatory sensations like feeling bugs crawling on or beneath the skin, often referred to as “meth mites.”
- Teeth Grinding
- Temporary Erectile Dysfunction
- Vasoconstriction
- Pupil Dilation
- Vibrating Vision: At high doses or specific routes of administration, the eyeballs may spontaneously tremble, causing blurry and temporarily unfocused vision, known as nystagmus.
- Seizure: Although uncommon, it can occur, especially in predisposed individuals or under physically demanding conditions.
Visual:
- The visual effects of methamphetamine tend to be inconsistent and generally mild, becoming more noticeable at higher doses. They are somewhat reminiscent of deliriants and are more prevalent in low-light settings. Severe sleep deprivation can intensify these effects, potentially leading to hallucinations.
Cognitive:
- The cognitive effects of methamphetamine intensify proportionally with dosage, marked by extreme mental stimulation, heightened focus, ego inflation, and potent euphoria. These align with typical stimulant cognitive effects. However, adverse side effects may manifest at higher doses or with widespread usage, particularly during the offset of the experience. Key cognitive products include:
- Analysis Enhancement
- Compulsive Redosing
- Ego Inflation
- Cognitive Euphoria: Often intense compared to other dopaminergic stimulants.
- Empathy, Affection, and Sociability Enhancement: Generally mild to moderate and tends to diminish with repeated use or tolerance.
- Focus Enhancement: Most effective at lower to moderate doses, as higher doses may impair concentration.
- Immersion Enhancement
- Increased Libido
- Increased Music Appreciation
- Memory Enhancement
- Motivation Enhancement
- Thought Acceleration
- Thought Organization
- Time Compression: Perceived acceleration of time.
- Wakefulness
After:
- The aftermath of a stimulant experience is often uncomfortable and harmful compared to the peak effects. Termed the “comedown,” it results from neurotransmitter depletion and may involve:
- Anxiety
- Appetite Suppression
- Cognitive Fatigue
- Depression
- Irritability
- Motivation Suppression
- Sleep Paralysis: Some users may experience this after consuming methamphetamine.
- Suicidal Ideation
- Thought Deceleration
- Psychosis
- Wakefulness: Particularly pronounced compared to other commonly used stimulants.
There is substantial evidence indicating that long-term use of methamphetamine can lead to brain damage in humans, resulting in adverse alterations in brain structure and function. These changes include reductions in grey matter volume in various brain regions and unfavourable shifts in markers of metabolic integrity.
Toxicity
Neurotoxicity:
In contrast to amphetamine, methamphetamine directly inflicts neurotoxicity upon dopamine neurons. Moreover, the abuse of methamphetamine elevates the risk of Parkinson’s disease due to excessive pre-synaptic dopamine autoxidation, a mechanism associated with neurotoxicity. Like its effects on the dopamine system, methamphetamine can also induce neurotoxicity in serotonin neurons. Notably, an elevated core temperature has been linked to increased neurotoxic effects of methamphetamine.
Due to methamphetamine-induced neurotoxicity to dopamine neurons, chronic use may result in post-acute withdrawals extending beyond the initial withdrawal phase, lasting for months or up to a year.
Dependence and Abuse Potential:
Like other stimulants, chronic methamphetamine use is highly addictive and carries a significant risk of abuse, often leading to psychological dependence among certain users. Individuals addicted may experience cravings and withdrawal symptoms if they abruptly cease usage.
Tolerance to methamphetamine develops rapidly with prolonged and repeated use, necessitating increasingly larger doses to achieve the same effects. After discontinuing use, it takes approximately 3 to 7 days for the tolerance to reduce by half and 1 to 2 weeks to return to baseline (assuming no further consumption). Methamphetamine induces cross-tolerance with all dopaminergic stimulants, meaning that the effects of other stimulants are diminished after methamphetamine use.
Research on effective treatments for amphetamine and methamphetamine dependence and abuse is limited. While some benefit has been observed with fluoxetine and imipramine in treating abuse and addiction, “no treatment has been demonstrated to be effective for the treatment of methamphetamine dependence and abuse.”
In heavily dependent amphetamine and methamphetamine users, “abrupt cessation of methamphetamine use in chronic, high-dose users often leads to a time-limited withdrawal syndrome within 24 hours of their last dose”. Withdrawal symptoms, which affect up to 87.6% of cases, persist for three to four weeks and feature a marked “crash” phase during the first week. Methamphetamine withdrawal symptoms encompass anxiety, drug cravings, dysphoric mood, fatigue, increased or decreased movement, increased appetite, sleep disturbances, vivid or lucid dreams, and lack of motivation. The mental depression associated with methamphetamine withdrawal surpasses that of cocaine withdrawal in duration and severity.
It remains debatable whether vaporized methamphetamine is inherently more addictive than oral or insufflated amphetamine despite being acknowledged as having a longer duration of action. The primary difference between the two lies in the proportional central and peripheral activity of methamphetamine, driven by its increased lipid solubility and the higher release of dopamine at equivalent doses compared to amphetamine. D-methamphetamine releases dopamine and norepinephrine at a ratio of approximately 1:1.3 from synapses, while d-amphetamine exhibits a balance of roughly 1:2 for dopamine and norepinephrine. D-methamphetamine also has a slight serotonergic effect, although this difference is likely negligible, given the low serotonin: norepinephrine release ratio of 1:60 for d-methamphetamine and 1:80 for d-amphetamine. Both drugs have minimal affinity for the serotonin transporter (SERT).
This increased central versus peripheral effect of methamphetamine aligns with the subjective feeling among stimulant users that methamphetamine’s high lacks an inherently ‘jittery’ quality. However, this aversive effect may serve as a deterrent against excessive use, although its real-world impact remains unclear. A small double-masked study involving 13 methamphetamine users showed only a slight preference for methamphetamine, possibly due to the users’ greater familiarity with the drug.
Harm Reduction:
Studies have demonstrated that N-acetylcysteine (NAC) can mitigate the harmful neurotoxic effects of methamphetamine while preventing neurotransmitter depletion in rats. Clinical trials to treat methamphetamine dependence in humans are ongoing, and NAC may also be effective in reducing cravings and psychological support. However, it’s essential to consider that this data is preliminary and may not directly apply to humans. Additionally, selenium has been shown to safeguard the brain against methamphetamine-induced neurotoxicity.
Dangerous Interactions:
Many psychoactive substances that are reasonably safe when used independently can become hazardous, even life-threatening when combined with specific other substances. The list below highlights some known dangerous interactions, although it may not cover all potential scenarios. It is crucial to conduct independent research (e.g., using Google, DuckDuckGo, PubMed) to ensure the safety of combining two or more substances. Some interactions are sourced from TripSit.
- Alcohol: Combining alcohol with stimulants is risky because it diminishes alcohol’s sedative effects, leading to excessive drinking and increased risk of liver damage and dehydration. Stimulants can also mask the usual signs of drunkenness, potentially leading to overconsumption. It’s advisable to limit alcohol intake and stick to it.
- GHB/GBL: Stimulants increase respiration rate, potentially leading to higher sedative doses. If the stimulant wears off before the depressant, respiratory arrest may occur.
- Opioids: Stimulants raise respiration rates, allowing higher opiate doses. If the stimulant effect wanes first, the opiate may overpower the user, causing respiratory arrest.
- Cocaine: Combining cocaine and amphetamine may result in cardiac effects due to serotonin-related valvulopathy from the activation of 5-HT2B. Amphetamines can cause hypertension, and this combination increases the risk of syncope due to turbulent blood flow during valve operation. Additionally, amphetamines reverse the rewarding mechanisms of cocaine.
- Cannabis: Stimulants can elevate anxiety levels and induce thought loops and paranoia, leading to negative experiences.
- Caffeine: Combining these stimulants is considered unnecessary and may strain the heart, causing anxiety and physical discomfort.
- Tramadol: Both substances raise heart rate, increasing the risk of seizures.
- DXM: DXM and stimulants elevate heart rate, potentially causing panic attacks and more severe heart issues in extreme cases.
- Ketamine: Combining amphetamine and ketamine may lead to psychosis resembling schizophrenia, mainly characterized by thought disorders and positive symptoms.
- PCP: Increases the risk of tachycardia, hypertension, and manic states.
- Methoxetamine: Elevates the risk of tachycardia, hypertension, and manic states.
- Psychedelics (e.g., LSD, mescaline, psilocybin): Raise the risk of anxiety, paranoia, and thought loops.
- 25x-NBOMe: Stimulants and NBOMes can result in tachycardia, hypertension, vasoconstriction, and, in extreme cases, heart failure.
- 2C-T-x and 5-MeO-xxT: Suspected of having mild MAOI properties, potentially increasing the risk of hypertensive crisis.
- DOx: Interactions with aMT, which has MAOI properties, may lead to unfavourable outcomes when combined with amphetamines.
- MAOIs: MAO-B inhibitors can unpredictably enhance the potency and duration of phenethylamines. MAO-A inhibitors, combined with amphetamine, can trigger hypertensive crises.
Legal status
The production, distribution, sale, and possession of methamphetamine are subject to strict legal restrictions or are illegal in numerous jurisdictions worldwide. Methamphetamine is classified as a controlled substance and regulated under international and national laws. Here are some examples of its legal status in various countries:
Australia: Methamphetamine is categorized under Schedule 8, allowing for medical use under authorized circumstances. However, possession, production, or supply without appropriate authorization is illegal.
Austria: Methamphetamine is illegal to possess, produce, and sell under the Austrian Suchtmittelgesetz (SMG), which deals with controlled substances.
Canada: Methamphetamine is listed as a Schedule I substance under the Controlled Drugs and Substances Act (CDSA), making it illegal to possess or distribute.
Germany: Methamphetamine was included in the Opium Act on July 1, 1941. It is controlled under Anlage II BtMG (Narcotics Act, Schedule II) as of March 1, 2008. Before this change, it could be prescribed with a narcotic prescription form when listed under Anlage III (Schedule III). Unauthorized activities such as manufacturing, possession, import, export, purchase, sale, procurement, or dispensing are prohibited without a license.
The Netherlands: Methamphetamine is classified as a List I controlled substance, subject to stringent regulations.
Switzerland: Methamphetamine is named explicitly as a controlled substance under Verzeichnis A, a list of controlled substances in Swiss law.
United Kingdom: Methamphetamine was classified as a Class A drug effective January 18, 2007, making its possession, production, and distribution illegal under UK law.
Czech Republic: Methamphetamine is designated as a Schedule II controlled substance in the Czech Republic, subject to strict legal controls and regulations.
It is crucial to note that these legal frameworks may change over time, and specific regulations can vary within each country’s jurisdictions. Therefore, it is essential to stay informed about local laws and regulations regarding methamphetamine.
FAQ
1. What is methamphetamine?
Methamphetamine, often called “meth,” is a powerful synthetic stimulant drug that affects the central nervous system. It is a member of the amphetamine class of drugs and is known for its stimulating and euphoric effects.
2. How is methamphetamine different from amphetamine?
Methamphetamine is structurally related to amphetamine but has an additional methyl group (CH3) in its structure. This structural difference makes methamphetamine more potent and leads to differences in its effects and potential risks.
3. How is methamphetamine used?
Methamphetamine can be used in various forms, including as a pill, powder, crystal, or white, odourless, bitter-tasting crystalline powder that can be ingested, snorted, smoked, or injected.
4. What are the short-term effects of methamphetamine use?
Short-term effects of methamphetamine use can include increased energy, alertness, and a sense of euphoria. Users may also experience decreased appetite, increased heart rate, and improved focus.
5. What are the long-term effects of methamphetamine use?
Long-term use of methamphetamine can lead to severe health problems, including addiction, dental issues (often called “meth mouth”), weight loss, insomnia, anxiety, paranoia, and cognitive impairment. It can also cause heart problems, skin infections, and damage to vital organs.
6. Is methamphetamine addictive?
Yes, methamphetamine is highly addictive. Its intense euphoric effects can lead to compulsive use and physical dependence. Quitting methamphetamine can be challenging due to withdrawal symptoms and intense cravings.
7. Are there any medical uses for methamphetamine?
Methamphetamine is rarely prescribed for medical use. In the United States, it is approved under the trade name “Desoxyn” for treating severe obesity and attention deficit hyperactivity disorder (ADHD) when other treatments have failed. Its medical use is strictly controlled due to its potential for abuse.
8. What are the legal consequences of methamphetamine possession and distribution?
The possession, distribution, sale, and production of methamphetamine are illegal in most countries. Legal consequences can include fines, imprisonment, and a criminal record.
9. How can I get help for methamphetamine addiction?
If you or someone you know is struggling with methamphetamine addiction, seeking professional help is crucial. Treatment options may include counselling, behavioural therapy, support groups, and, in some cases, medication-assisted treatment. Reach out to a healthcare provider or addiction specialist for guidance.
10. What are the dangers of methamphetamine use?
Methamphetamine use carries significant risks, including addiction, physical and mental health problems, overdose, and even death. It can also lead to risky behaviours and strained relationships.
11. Is there any harm reduction advice for methamphetamine users?
Harm reduction practices, such as using clean needles and not sharing injection equipment, can reduce the risks associated with methamphetamine use. Additionally, seeking medical help for any health issues related to methamphetamine use is crucial.
12. Can methamphetamine use lead to mental health issues?
Long-term methamphetamine use is associated with mental health problems, including anxiety, paranoia, hallucinations, and even psychosis. It can also exacerbate pre-existing mental health conditions.
13. Is there any research on the medical benefits of methamphetamine?
While methamphetamine has some limited medical uses, its potential benefits must be carefully weighed against its significant risks. Research is ongoing, but its use remains controversial due to its potential for abuse and addiction.
Remember that using methamphetamine is illegal in many places and can have severe consequences for your health. If you or someone you know is struggling with methamphetamine use, seek help from a healthcare professional or addiction support organization.
References
- Rau, T., Ziemniak, J., Poulsen, D. (January 4 2016) explored “The neuroprotective potential of low-dose methamphetamine in preclinical models of stroke and traumatic brain injury” in the journal Progress in Neuro-Psychopharmacology and Biological Psychiatry (Vol. 64, pp. 231–236). DOI: 10.1016/j.pnpbp.2015.02.013. ISSN: 0278-5846.
- Barr, A. M., Panenka, W. J., MacEwan, G. W., Thornton, A. E., Lang, D. J., Honer, W. G., Lecomte, T. (September 2006) provided “The need for speed: an update on methamphetamine addiction” in the Journal of Psychiatry and Neuroscience (Vol. 31, No. 5, pp. 301–313). ISSN: 1180-4882.
- Nagai N (1893) conducted “Studies on the components of Ephedraceae in herb medicine” in the Yakugaku Zasshi (Vol. 139, pp. 901-933).
- Galbraith, N. (October 2015) discussed “The methamphetamine problem” in BJPsych Bulletin (Vol. 39, No. 5, pp. 218–220). DOI: 10.1192/pb.bp.115.050930. ISSN: 2056-4694.
- Jayanthi, S., Daiwile, A. P., Cadet, J. L. (October 2021) delved into the “Neurotoxicity of methamphetamine: Main effects and mechanisms” in the journal Experimental Neurology (Vol. 344). DOI: 10.1016/j.expneurol.2021.113795. ISSN: 0014-4886.
- Khoshsirat, S., Khoramgah, M. S., Mahmoudiasl, G.-R., Rezaei-Tavirani, M., Abdollahifar, M.-A., Tahmasebinia, F., Darabi, S., Niknazar, S., Abbaszadeh, H. A. (September 2020) explored “LC3 and ATG5 overexpression and neuronal cell death in the prefrontal cortex of postmortem chronic methamphetamine users” in the Journal of Chemical Neuroanatomy (Vol. 107). DOI: 10.1016/j.jchemneu.2020.101802. ISSN: 0891-0618.
- Edeleano, L. (January 1887) discussed “Ueber einige Derivate der Phenylmethacrylsäure und der Phenylisobuttersäure” in Berichte der deutschen chemischen Gesellschaft (Vol. 20, No. 1, pp. 616–622). DOI: 10.1002/cber.188702001142. ISSN: 0365-9496.
- Grobler, S. R., Chikte, U., Westraat, J. (June 26 2011) investigated “The pH Levels of Different Methamphetamine Drug Samples on the Street Market in Cape Town” in ISRN Dentistry (Vol. 2011, pp. 1–4). DOI: 10.5402/2011/974768. ISSN: 2090-4371.
- Rasmussen, N. (February 21 2006) delved into “Making the First Anti-Depressant: Amphetamine in American Medicine, 1929-1950” in the Journal of the History of Medicine and Allied Sciences (Vol. 61, No. 3, pp. 288–323). DOI: 10.1093/jhmas/jrj039. ISSN: 0022-5045.
- Rasmussen, N. (September 2011) discussed “Medical Science and the Military: The Allies’ Use of Amphetamine during World War II” in The Journal of Interdisciplinary History (Vol. 42, No. 2, pp. 205–233). DOI: 10.1162/JINH_a_00212. ISSN: 0022-1953.
- Defalque, R. J., Wright, A. J. (April 2011) explored “Methamphetamine for Hitler’s Germany: 1937 to 1945” in the Bulletin of Anesthesia History (Vol. 29, No. 2, pp. 21–32). DOI: 10.1016/S1522-8649(11)50016-2. ISSN: 1522-8649.
- The “Historical overview of methamphetamine” was provided by the Vermont Department of Health, Government of Vermont. Retrieved on January 29 2012.
- “Controlled Substances Act” was detailed by the United States Food and Drug Administration on June 11 2009. Retrieved on November 4 2013.
- Gyenis A. discussed “Forty Years of On the Road 1957–1997” on wordsareimportant.com, DHARMA beat. Archived from the original on February 14 2008. Retrieved on March 18 2008.
- Wilson, A. (2009) examined “Mixing the Medicine: The Unintended Consequence of Amphetamine Control on the Northern Soul Scene” in Social Science Research Network.
- Hill, J. (2004) explored “Paul Erdős – Mathematical Genius, Human (In That Order)”.
- Liddle, D. G., Connor, D. J. (June 2013) discussed “Nutritional Supplements and Ergogenic Aids” in Primary Care: Clinics in Office Practice (Vol. 40, No. 2, pp. 487–505). DOI: 10.1016/j.pop.2013.02.009. ISSN: 0095-4543.
- Chawla S, Le Pichon T (2006) provided information in the “World Drug Report 2006” by the United Nations Office on Drugs and Crime (pp. 128–135). Retrieved on November 2 2013.
- “Desoxyn Label (FDA)” is available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/005378s028lbl.pdf.
- Kish, S. J. (June 17 2008) examined the “Pharmacologic mechanisms of crystal meth” in CMAJ: Canadian Medical Association Journal (Vol. 178, No. 13, pp. 1679–1682). DOI: 10.1503/cmaj.071675. ISSN: 0820-3946.
- Haughey, H. M., Brown, J. M., Wilkins, D. G., Hanson, G. R., Fleckenstein, A. E. (April 28 2000) explored the “Differential effects of methamphetamine on Na(+)/Cl(-)-dependent transporters” in Brain Research (Vol. 863, No. 1–2, pp. 59–65). DOI: 10.1016/s0006-8993(00)02094-1. ISSN: 0006-8993.
- Lin, M., Sambo, D., Khoshbouei, H. (October 5 2016) investigated “Methamphetamine Regulation of Firing Activity of Dopamine Neurons” in The Journal of Neuroscience: The Official Journal of the Society for Neuroscience (Vol. 36, No. 40, pp. 10376–10391). DOI: 10.1523/JNEUROSCI.1392-16.2016. ISSN: 1529-2401.
- “How Drugs Affect Neurotransmitters” was discussed by the Canadian Institutes of Health Research, retrieved on January 1, 2007.
- Nie, L., Zhao, Z., Wen, X., Luo, W., Ju, T., Ren, A., Wu, B., Li, J. (April 10 2020) examined “Gray-matter structure in long-term abstinent methamphetamine users” in BMC Psychiatry (Vol. 20, No. 1, p. 158). DOI: 10.1186/s12888-020-02567-3. ISSN: 1471-244X.
- Nestler, E. J., Hyman, S. E., Malenka, R. C. (2009) discussed “Molecular neuropharmacology: a foundation for clinical neuroscience” (2nd ed.) published by McGraw-Hill Medical. ISBN: 9780071481274.
- Cruickshank, C. C., Dyer, K. R. (July 2009) provided a review of “A review of the clinical pharmacology of methamphetamine” in Addiction (Abingdon, England) (Vol. 104, No. 7, pp. 1085–1099). DOI: 10.1111/j.1360-0443.2009.02564.x. ISSN: 1360-0443.
- Thrash, B., Thiruchelvan, K., Ahuja, M., Subramaniam, V., Dhanasekaran, M. (November 2009) examined “Methamphetamine-induced neurotoxicity: the road to Parkinson’s disease” in Pharmacological Reports (Vol. 61, No. 6, pp. 966–977). DOI: 10.1016/S1734-1140(09)70158-6. ISSN: 1734-1140.
- Sulzer, D., Zecca, L. (February 2000) discussed “Intraneuronal dopamine-quinone synthesis: a review” in Neurotoxicity Research (Vol. 1, No. 3, pp. 181–195). DOI: 10.1007/BF03033289. ISSN: 1029-8428.
- Miyazaki, I., Asanuma, M. (June 2008) examined “Dopaminergic neuron-specific oxidative stress caused by dopamine itself” in Acta Medica Okayama (Vol. 62, No. 3, pp. 141–150). DOI: 10.18926/AMO/30942. ISSN: 0386-300X.
- Krasnova, I. N., Cadet, J. L. (May 2009) discussed “Methamphetamine toxicity and messengers of death” in Brain Research Reviews (Vol. 60, No. 2, pp. 379–407). DOI: 10.1016/j.brainresrev.2009.03.002. ISSN: 0165-0173.
- Yuan, J., Hatzidimitriou, G., Suthar, P., Mueller, M., McCann, U., Ricaurte, G. (March 2006) explored the “Relationship between temperature, dopaminergic neurotoxicity, and plasma drug concentrations in methamphetamine-treated squirrel monkeys” in The Journal of Pharmacology and Experimental Therapeutics (Vol. 316, No. 3, pp. 1210–1218). DOI: 10.1124/jpet.105.096503. ISSN: 0022-3565.
- Pérez-Mañá, C., Castells, X., Torrens, M., Capellà, D., Farre, M. (September 2 2013) examined the “Efficacy of psychostimulant drugs for amphetamine abuse or dependence” in The Cochrane Database of Systematic Reviews (No. 9). DOI: 10.1002/14651858.CD009695.pub2. ISSN: 1469-493X.
- “Merck Manuals” provides information on amphetamines. Retrieved from http://www.merckmanuals.com/home/special_subjects/drug_use_and_abuse/amphetamines.html.
- Srisurapanont, M., Jarusuraisin, N., Kittirattanapaiboon, P. (2001) examined “Treatment for amphetamine dependence and abuse” in The Cochrane Database of Systematic Reviews (No. 4). DOI: 10.1002/14651858.CD003022. ISSN: 1469-493X.
- Shoptaw, S. J., Kao, U., Heinzerling, K., Ling, W. (April 15 2009) discussed “Treatment for amphetamine withdrawal” in The Cochrane Database of Systematic Reviews (No. 2). DOI: 10.1002/14651858.CD003021.pub2. ISSN: 1469-493X.
- Winslow, B. T., Voorhees, K. I., Pehl, K. A. (October 15 2007) explored “Methamphetamine abuse” in the American Family Physician (Vol. 76, No. 8, pp. 1169–1174). ISSN: 0002-838X.
- “Monoamine releasing agent” activity profiles can be found at https://en.wikipedia.org/wiki/Monoamine_releasing_agent#Activity_profiles.
- Kirkpatrick, M. G., Gunderson, E. W., Johanson, C.-E., Levin, F. R., Foltin, R. W., Hart, C. L. (April 2012) compared “Intranasal methamphetamine and d-amphetamine self-administration by humans” in Addiction (Abingdon, England) (Vol. 107, No. 4, pp. 783–791). DOI: 10.1111/j.1360-0443.2011.03706.x. ISSN: 0965-2140.
- Hofmann, F. G. (1983) provided information in “A handbook on drug and alcohol abuse: the biomedical aspects” (2nd ed.) published by Oxford University Press. ISBN: 9780195030563.
- “Desoxyn Prescribing Information” is available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/005378s028lbl.pdf.
- Goodman, L. S., Brunton, L. L., Chabner, B., Knollmann, B. C., eds. (2011) published “Goodman & Gilman’s pharmacological basis of therapeutics” (12th ed.) by McGraw-Hill. ISBN: 9780071624428.
- “Desoxyn Prescribing Information” is available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/005378s028lbl.pdf.
- Fukami, G., Hashimoto, K., Koike, K., Okamura, N., Shimizu, E., Iyo, M. (July 2004) examined the “Effect of antioxidant N-acetyl-l-cysteine on behavioural changes and neurotoxicity in rats after administration of methamphetamine” in Brain Research (Vol. 1016, No. 1, pp. 90–95). DOI: 10.1016/j.brainres.2004.04.072. ISSN: 0006-8993.
- Mousavi, S. G., Sharbafchi, M. R., Salehi, M., Peykanpour, M., Karimian Sichani, N., Maracy, M. (January 2015) explored “The efficacy of N-acetylcysteine in the treatment of methamphetamine dependence: a double-blind controlled, crossover study” in the Archives of Iranian Medicine (Vol. 18, No. 1, pp. 28–33). DOI: 10.1016/j.brainres.2004.04.072. ISSN: 1735-3947.
- Imam, S. Z., Newport, G. D., Islam, F., Slikker, W., Ali, SF. (February 13 1999) “Selenium, an antioxidant, protects against methamphetamine-induced dopaminergic neurotoxicity” in Brain Research (Vol. 818, No. 2, pp. 575–578). DOI: 10.1016/S0006-8993(98)01311-0. ISSN: 0006-8993.
- Greenwald, M. K., Lundahl, L. H., Steinmiller, C. L. (December 2010) explored “Sustained Release d-Amphetamine Reduces Cocaine but not ‘Speedball’-Seeking in Buprenorphine-Maintained Volunteers: A Test of Dual-Agonist Pharmacotherapy for Cocaine/Heroin Polydrug Abusers” in Neuropsychopharmacology (Vol. 35, No. 13, pp. 2624–2637). DOI: 10.1038/npp.2010.175. ISSN: 0893-133X.
- Siciliano, CA., Saha, K., Calipari, E. S., Fordahl, S. C., Chen, R., Khoshbouei, H., Jones, SR. (January 10 2018) examined “Amphetamine Reverses Escalated Cocaine Intake via Restoration of Dopamine Transporter Conformation” in The Journal of Neuroscience (Vol. 38, No. 2, pp. 484–497). DOI: 10.1523/JNEUROSCI.2604-17.2017. ISSN: 0270-6474.
- Krystal, J. H., Perry, E. B., Gueorguieva, R., Belger, A., Madonick, S. H., Abi-Dargham, A., Cooper, T. B., MacDougall, L., Abi-Saab, W., D’Souza, D. C. (September 1 2005) compared and examined “Comparative and Interactive Human Psychopharmacologic Effects of Ketamine and Amphetamine: Implications for Glutamatergic and Dopaminergic Model Psychoses and Cognitive Function” in the Archives of General Psychiatry (Vol. 62, No. 9, pp. 985). DOI: 10.1001/archpsyc.62.9.985. ISSN: 0003-990X.
- “World Drug Report 2006” was provided by the United Nations Office on Drugs and Crime (pp. 128–135).
- “The Nazi Death Machine: Hitler’s Drugged Soldiers” was discussed in Spiegel Online on May 6, 2005.
- “Anlage II BtMG” (in German) is available on the Bundesministerium der Justiz und für Verbraucherschutz website.
- “Einundzwanzigste Verordnung zur Änderung betäubungsmittelrechtlicher Vorschriften” (in German) is available on the Bundesanzeiger Verlag website.
- “§ 29 BtMG” (in German) is available on the Bundesministerium der Justiz und für Verbraucherschutz website.
- “Desoxyn Label (FDA)” is available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/005378s028lbl.pdf.
- “Merck Manuals” provides information on amphetamines. Retrieved from http://www.merckmanuals.com/home/special_subjects/drug_use_and_abuse/amphetamines.html.
- “Controlled Substances Act” was detailed by the United States Food and Drug Administration on June 11 2009. Retrieved on November 4 2013.