Contents
- 1 Summary
- 2 Pharmacology
- 3 Metabolism
- 4 Synthesis
- 5 Legal status
- 6 FAQ
- 6.1 1. What is Methiopropamine (MPA)?
- 6.2 2. Is Methiopropamine legal?
- 6.3 3. What are the effects of Methiopropamine?
- 6.4 4. Is Methiopropamine safe to use?
- 6.5 5. How is Methiopropamine metabolized in the body?
- 6.6 6. Why has Methiopropamine been subject to legal restrictions?
- 6.7 7. Can I legally possess or use Methiopropamine for research purposes?
- 6.8 8. Are there any ongoing regulatory changes related to Methiopropamine?
- 7 References
Summary
Methiopropamine (MPA) is an organic compound that shares a structural resemblance with methamphetamine. First documented in 1942, this molecule features a thiophene group with an alkyl amine substituent at the 2-position. It entered the public market in the United Kingdom in December 2010, marketed as a “research chemical” or “legal high” and more recently known as “Blow.” However, it has only gained modest popularity as a recreational stimulant.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 801156-47-8 7464-94-0 (hydrochloride) |
|---|---|
| PubChem CID | 436156 |
| ChemSpider | 385727 |
| UNII | 64ON2ETH7I |
| Chemical and physical data | |
| Formula | C8H13NS |
| Molar mass | 155.26 g·mol−1 |
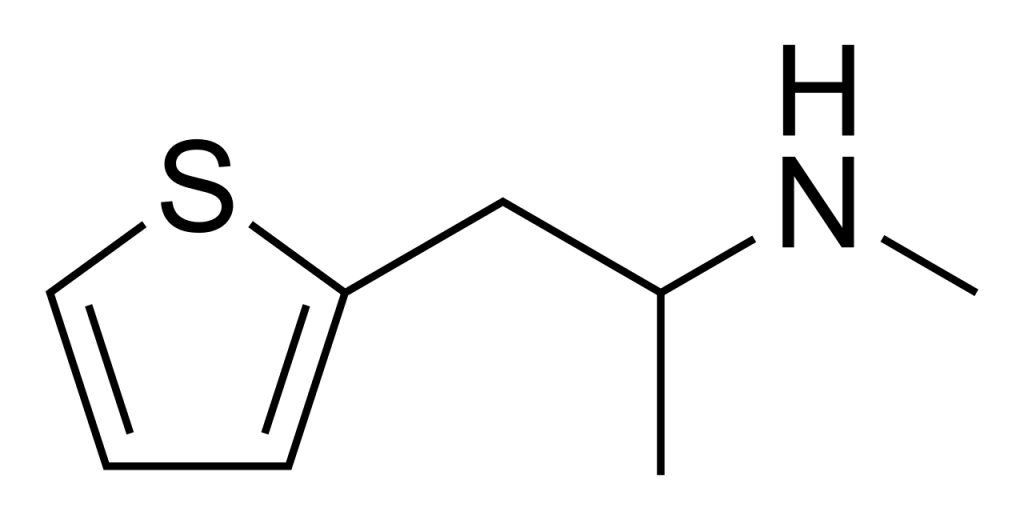
Pharmacology
Methiopropamine operates as a norepinephrine-dopamine reuptake inhibitor, demonstrating approximately 1.85 times greater selectivity for norepinephrine over dopamine. Its potency as a norepinephrine reuptake inhibitor is approximately one-third that of dextroamphetamine, and it is about one-fifth as effective as a dopamine reuptake inhibitor. Notably, it exhibits minimal activity as a serotonin reuptake inhibitor.
It’s crucial to recognize that Methiopropamine carries the potential for considerable acute toxicity, manifesting in cardiovascular, gastrointestinal, and psychotic symptoms.
Metabolism
In the case of N-alkyl amphetamines, the primary elimination pathways involve deamination and N-dealkylation, with renal excretion playing a minor role.
The metabolism of Methiopropamine leads to the formation of active compounds, including thiopropamine, 4-hydroxymethiopropamine, and thiophene S-oxides. These metabolites, which undergo N-demethylation, are further subjected to deamination in the liver, facilitated by the cytochrome P450 enzyme CYP2C19. This process transforms them into an inactive compound known as 1-(thiophen-2-yl)-2-propan-2-one, which can be regarded as a derivative of phenylacetone.
Ultimately, the major end product of metabolism is Thiophene-2-carboxylic acid, which is highly hydrophilic and is excreted through urine. Methiopropamine, and to a greater extent, thiopropamine, are also excreted in their unchanged forms.
Synthesis
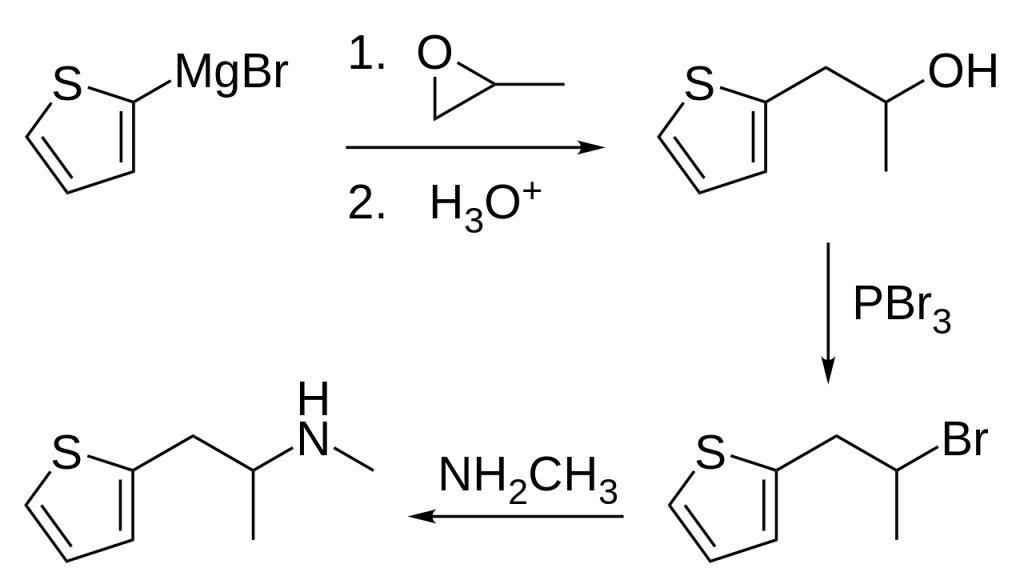
The synthesis of methiopropamine involves a four-step process. It commences with the reaction of (thiophen-2-yl)magnesium bromide with propylene oxide, resulting in the formation of 1-(thiophen-2-yl)-2-hydroxypropane. Subsequently, this compound is subjected to a reaction with phosphorus tribromide, leading to the production of 1-(thiophen-2-yl)-2-bromopropane. Finally, the last step involves the reaction of 1-(thiophen-2-yl)-2-bromopropane with methylamine, culminating in the synthesis of 1-(thiophen-2-yl)-2-methylaminopropane.
Legal status
China: As of October 2015, Methiopropamine (MPA) is classified as a controlled substance in China.
Finland: The possession and use of Methiopropamine are prohibited in Finland.[citation needed]
Germany: Methiopropamine is explicitly illegal in Germany.
United Kingdom: Methiopropamine came under scrutiny in the United Kingdom following the ban on ethylphenidate. Authorities observed an increase in its use, particularly among injection drug users. On November 18, 2015, the Advisory Council on the Misuse of Drugs (ACMD) recommended its prohibition due to its similar effects to methylphenidate. The government promptly enacted a temporary drug control order a week later, which became effective on November 27, 2015. While such orders usually last for one year, the ACMD reported a significant decline in MPA use since its implementation, making it challenging to gather information about the drug. Consequently, they requested a one-year extension of the control order, which was granted.[17] Methiopropamine was ultimately classified as a Class B controlled drug under the Misuse of Drugs Act 1971 (as amended) (Amendment)(No.2) Order 2017 [SI 2017/1114], effective from November 27, 2017.
United States: Methiopropamine is categorized as a Schedule I controlled substance at the federal level in the United States. The Drug Enforcement Administration (DEA) had initially proposed placing methiopropamine in Schedule I of Controlled Substances and sought public input until October 4, 2021. Subsequently, it was officially included in Schedule I.
Florida: In the state of Florida, Methiopropamine is classified as a Schedule I controlled substance. This designation makes it illegal to purchase, sell, or possess Methiopropamine in Florida.
Tasmania, Australia: Methiopropamine is considered a “controlled substance” in Tasmania, Australia. Consequently, it is deemed an “illegal drug” to import, possess, or engage in any form of sale or trafficking without explicit authorization from the relevant government agency.
FAQ
1. What is Methiopropamine (MPA)?
Methiopropamine, abbreviated as MPA, is an organic compound that shares structural similarities with methamphetamine. It is categorized as a research chemical and has been used for its stimulant properties.
2. Is Methiopropamine legal?
The legal status of Methiopropamine varies by country and jurisdiction. It is explicitly illegal in several countries, including the United Kingdom, Germany, and Finland. In the United States, it is classified as a Schedule I controlled substance at the federal level.
3. What are the effects of Methiopropamine?
Methiopropamine is known to function as a norepinephrine-dopamine reuptake inhibitor. It may produce stimulant effects and increase the levels of certain neurotransmitters in the brain, leading to heightened alertness and energy. However, its recreational use is associated with potential health risks and adverse effects.
4. Is Methiopropamine safe to use?
The safety of Methiopropamine is a subject of concern. Limited information is available about its safety profile, and its use has been associated with adverse effects, including cardiovascular, gastrointestinal, and psychotic symptoms. Its unregulated nature and lack of clinical testing make it a potentially risky substance.
5. How is Methiopropamine metabolized in the body?
Methiopropamine undergoes metabolic processes in the body, leading to the formation of various metabolites. These metabolites include thiopropamine, 4-hydroxymethiopropamine, and thiophene S-oxides. The metabolism involves N-demethylation and deamination processes, primarily occurring in the liver.
6. Why has Methiopropamine been subject to legal restrictions?
Methiopropamine has been classified as a controlled substance or banned in several countries due to concerns about its safety, potential for abuse, and adverse health effects. Regulatory agencies and governments have taken action to control its availability and use.
7. Can I legally possess or use Methiopropamine for research purposes?
The legality of possessing and using Methiopropamine for research purposes varies by jurisdiction. Researchers should consult local laws and regulations governing the possession and use of research chemicals to ensure compliance.
Regulatory changes related to Methiopropamine can occur, with governments periodically reviewing the legal status of substances. Researchers and individuals should stay informed about any updates in regulations pertaining to Methiopropamine in their respective regions.
References
- Anvisa (Agência Nacional de Vigilância Sanitária) – In July 2023, Anvisa issued a resolution (RDC Nº 804) in Brazil, listing Methiopropamine as a controlled substance, categorizing it under substances with special control.
- Early Discovery – Methiopropamine’s discovery dates back to March 1942 when Blicke FF and Burckhalter JH first reported it in a scientific journal as α-Thienylaminoalkanes.
- Synthesis Reference – The synthesis of Methiopropamine has been documented. Researchers Angelov D, O’Brien J, and Kavanagh P published a method for synthesizing it, which has been used as a reference standard in studies.
- UKChemicalResearch – Methiopropamine has been discussed in various online communities, including the UKChemicalResearch forum, where it is a subject of interest among researchers and enthusiasts.
- Neurochemical Profiles – Studies like the one by Iversen et al. (2013) have explored the neurochemical profiles of Methiopropamine and other novel psychoactive substances, shedding light on their pharmacological effects.
- Locomotor Sensitization – Research conducted by Yoon et al. (2016) investigated the locomotor sensitization induced by Methiopropamine, revealing the involvement of dopamine receptors in rats.
- Acute Toxicity – The acute toxicity associated with Methiopropamine use has been documented. Lee et al. (2014) reported cases of toxicity linked to recreational Methiopropamine consumption.
- Metabolism Studies – Methiopropamine’s metabolic pathways have been studied extensively. Welter et al. (2013) delved into its metabolism in both rats and humans using advanced analytical techniques.
- Regulatory Actions – Various countries have taken regulatory actions concerning Methiopropamine. For example, China classified it as a controlled substance in October 2015.
- UK Temporary Ban – In the United Kingdom, Methiopropamine came under scrutiny following an increase in its use after the ban on methylphenidate. The Advisory Council on the Misuse of Drugs (ACMD) recommended its ban in November 2015, and a temporary drug control order was enacted a week later.
- US Federal Scheduling – In the United States, Methiopropamine is scheduled at the federal level, with the Drug Enforcement Administration (DEA) accepting public comments on its classification until October 4, 2021. It was later placed in Schedule I.
- Florida State Ban – Methiopropamine is classified as a Schedule I controlled substance in the state of Florida, making it illegal to buy, sell, or possess within the state.
- Tasmania, Australia – Methiopropamine is considered a “controlled substance” in Tasmania, Australia, and is, therefore illegal to import, possess, or sell without proper government authorization.