Contents
- 1 Summary
- 2 Use in the United Kingdom
- 3 Pharmacology
- 4 FAQ
- 4.1 1. What is Naphyrone?
- 4.2 2. What are the effects of Naphyrone?
- 4.3 3. Is Naphyrone a legal substance?
- 4.4 4. What is the pharmacological profile of Naphyrone?
- 4.5 5. Are there safety concerns associated with Naphyrone use?
- 4.6 6. Can Naphyrone be detected in drug tests?
- 4.7 7. What is the prevalence of Naphyrone in designer drug products?
- 4.8 8. What is the legal history of Naphyrone?
- 4.9 9. What is the significance of α-pyrone in the context of Naphyrone use?
- 5 References
Summary
Naphyrone, alternatively referred to as O-2482 and naphthyl pyrovalerone, is categorized as a substituted cathinone substance derived from pyrovalerone. This compound functions as a triple reuptake inhibitor, resulting in stimulant effects, and it has emerged as a novel designer drug. It’s important to note that there is currently no available data regarding the safety or toxicity of this drug.
Naphyrone has been marketed under the moniker “NRG-1.” However, it’s worth mentioning that only a minority of the substances sold under this name have been confirmed to contain actual nephrons. Even in cases where genuine β-naphyrone was present, some samples also exhibited varying proportions of the 1-naphthyl isomer α-naphyrone. This complexity has added to the challenge of accurately defining the reported effects of this compound.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 850352-53-3 850352-11-3 (hydrochloride) |
|---|---|
| PubChem CID | 11243002 |
| ChemSpider | 9418039 |
| UNII | 96ON64182B |
| CompTox Dashboard (EPA) | DTXSID301014192 |
| Chemical and physical data | |
| Formula | C19H23NO |
| Molar mass | 281.399 g·mol−1 |
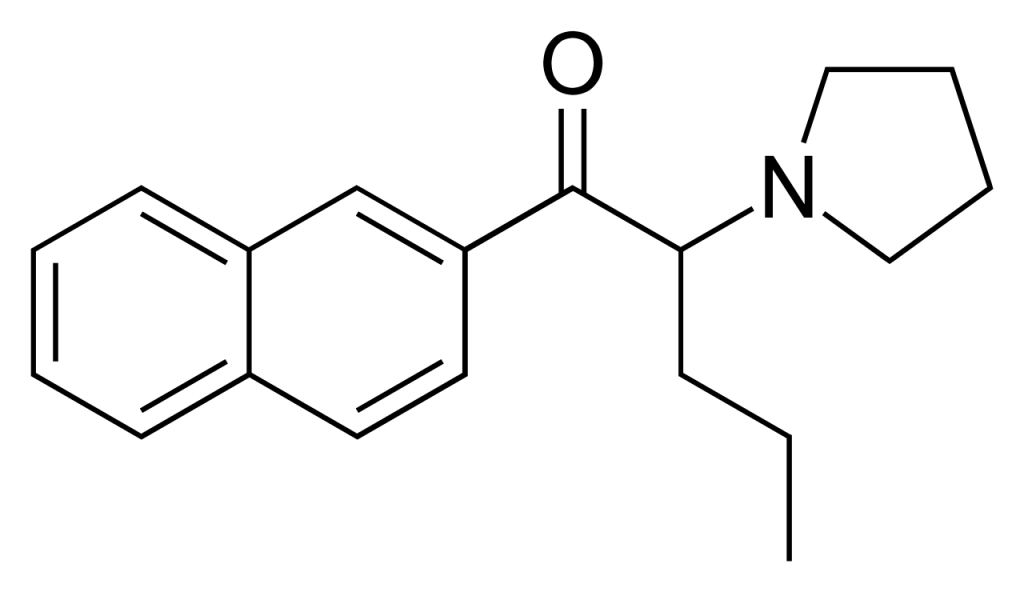
Use in the United Kingdom
Naphyrone came into prominence as a new legal high within the United Kingdom shortly after the prohibition of a similar drug, mephedrone, which also belonged to the cathinone derivative family. Prior to July 2010, this substance was not regulated under the Misuse of Drugs Act 1971, making it legal for individuals to possess. However, its sale for human consumption was prohibited by the Medicines Act. Consequently, it was sometimes marketed as ‘pond cleaner’ or under alternative names to evade legal restrictions.
A study conducted by researchers at Liverpool John Moores University revealed that merely one out of ten products labelled as “NRG-1” genuinely contained pyrone upon meticulous laboratory examination. Products bearing the NRG-1 label were found to contain a mixture of compounds, including MDPV, mephedrone, mephedrone butylene, and caffeine. In some instances, the composition of a tested product was inorganic. It’s noteworthy that if an individual possesses a product labelled as NRG-1 that contains MDPV or other illicit substances, they are, in fact, in possession of a controlled substance.
In response to these developments, on July 12, 2010, the Home Office took action to prohibit pyrone, categorizing it as a Class B drug. This decision was based on a recommendation from the Advisory Council on the Misuse of Drugs.
Pharmacology
In laboratory settings, pyrone has demonstrated its capacity as a triple reuptake inhibitor, impacting the reuptake processes of key neurotransmitters, namely serotonin, dopamine, and norepinephrine. This interaction occurs through its engagement with the serotonin transporter (SERT), dopamine transporter (DAT), and norepinephrine transporter (NET).
A specific study delved into the quantitative aspects of naphyrone’s interaction with these transporters. The dissociation constants for naphyrone’s binding to SERT were measured at 33.1nM ± 1.1, to DAT at 20.1nM ± 7.1, and to NET at 136nM ± 27. Furthermore, the concentration of naphyrone necessary to inhibit these transporters by 50% was determined to be 46.0nM ± 5.5 for SERT, 40.0nM ± 13 for DAT, and 11.7nM ± 0.9 for NET. Among the range of pyrovalerone analogues examined, pyrone stood out as the sole triple reuptake inhibitor active at nanomolar (nM) concentrations.
It is worth noting that certain samples of β-naphyrone available for sale have been found to contain the alternative isomer α-naphyrone. This presence of α-pyrone is presumed to be accidental and may result from impurities during synthesis. The scientific literature exclusively provides in vitro data concerning pure β-naphyrone, and the pharmacological characteristics of α-naphyrone remain unexplored. Consequently, this adds complexity to the overall pharmacological profile of this relatively understudied designer drug.

FAQ
1. What is Naphyrone?
- Naphyrone, also known as O-2482 and naphthylpyrovalerone, is a synthetic compound classified as a substituted cathinone. It acts as a triple reuptake inhibitor, affecting the reuptake of neurotransmitters such as serotonin, dopamine, and norepinephrine.
2. What are the effects of Naphyrone?
- Naphyrone produces stimulant effects due to its action on neurotransmitter reuptake. These effects can include increased alertness, energy, and mood elevation. However, the specific effects and their intensity may vary among individuals.
3. Is Naphyrone a legal substance?
- The legal status of Naphyrone varies by country and region. It may be regulated as a controlled substance in some places, while in others, it might be classified as a designer or synthetic drug.
4. What is the pharmacological profile of Naphyrone?
- Naphyrone is known for its ability to interact with serotonin transporters (SERT), dopamine transporters (DAT), and norepinephrine transporters (NET) as a triple reuptake inhibitor. This means it affects the reabsorption of these neurotransmitters, resulting in increased levels in the brain.
5. Are there safety concerns associated with Naphyrone use?
- Due to limited research, there is a lack of safety and toxicity data available for Naphyrone. As with many synthetic drugs, there are potential risks associated with its use, including addiction and adverse health effects.
6. Can Naphyrone be detected in drug tests?
- Naphyrone may not be included in standard drug tests. Detection may require specialized tests designed to identify synthetic cathinones.
7. What is the prevalence of Naphyrone in designer drug products?
- Studies have shown that products labelled as “NRG-1” often contain a mix of compounds, with only a minority of them actually containing Naphyrone. Some of these products have been found to contain alternative substances like MDPV, mephedrone, and caffeine.
8. What is the legal history of Naphyrone?
- Naphyrone gained attention as a legal high, particularly in the UK, following the ban on the similar drug mephedrone. It was initially unregulated but later classified as a Class B drug in the UK in July 2010.
9. What is the significance of α-pyrone in the context of Naphyrone use?
- Some samples of Naphyrone sold have been found to contain α-pyrone, likely as an impurity in synthesis. However, the pharmacological properties of α-pyrone are not well understood, which complicates the understanding of Naphyrone’s effects.
References
- The “Schedules of Controlled Substances: Temporary Placement of 10 Synthetic Cathinones Into Schedule I” document, archived as of April 14, 2015, highlights regulatory measures pertaining to synthetic cathinones. It was retrieved on April 5, 2015.
- The “Ustawa z dnia 15 kwietnia 2011 r. o zmianie ustawy o przeciwdziałaniu narkomanii” document, retrieved from the Internetowy System Aktów Prawnych, addresses changes in drug-related legislation in Poland. It was retrieved on June 17, 2011.
- A news article titled “Deadly New ‘Legal’ Drug Bound For Britain” discusses the emergence of a new legal drug, highlighting its potential dangers. The article was retrieved from Yahoo! News UK on April 3, 2010.
- In a study published in February 2006, Meltzer PC, Butler D, Deschamps JR, and Madras BK explore Pyrovalerone analogues as monoamine uptake inhibitors in the Journal of Medicinal Chemistry (doi:10.1021/jm050797a; PMC 2602954; PMID 16480278).
- An article from The Guardian published on April 1, 2010, discusses the potential ban on NRG-1, a legal high, by government ministers (Archived from the original on 2021-05-31).
- “The Herald” published an article on April 2, 2010, highlighting concerns about a new legal drug referred to as “50c legal drug” and its perceived risks (Archived from the original on 7 April 2010).
- Brandt SD, Sumnall HR, Measham F, and Cole J published a study in July 2010 discussing the confusing nature of NRG-1, a second-generation mephedrone (BMJ, doi:10.1136/bmj.c3564; PMID 20605894; S2CID 20354123).
- In August 2010, Brandt SD, Sumnall HR, Measham F, and Cole J published a paper titled “Analyses of second-generation ‘legal highs’ in the UK: initial findings” in Drug Testing and Analysis (doi:10.1002/dta.155; PMID 20687197).
- Wood DM, Davies S, Cummins A, and others, published a study in December 2011 discussing “Energy-1 (‘NRG-1’)” and its legal status and composition (Emergency Medicine Journal, doi:10.1136/emj.07.2010.3184rep; PMC 3062281; PMID 22101594).
- Brandt SD, Wootton RC, De Paoli G, and Freeman S published a paper in October 2010 exploring the question of whether Naphyrone exists in its alpha or beta-naphthyl isomer form (Drug Testing and Analysis, doi:10.1002/dta.185; PMID 20886463).
- “BBC News” reported on July 12, 2010, that NRG-1, a ‘legal high’ drug, was banned in the UK (Archived from the original on 2021-05-31).
- The “Advisory Council on the Misuse of Drugs Naphyrone Report (2010)” was issued by the Home Office on July 7, 2010, providing insights into Naphyrone’s classification and regulatory decisions (Archived from the original on 2010-07-17).
- “The Misuse of Drugs (Amendment No. 2) (England, Wales and Scotland) Regulations 2010 No. 1799” is a legal document outlining changes in drug regulations in the UK (PDF).
- The “Explanatory Memorandum To The Misuse of Drugs (Amendment No. 2) (England, Wales and Scotland) Regulations 2010 No. 1799” provides additional context regarding the regulatory changes (PDF).
- A duplicate reference to Brandt SD, Wootton RC, De Paoli G, and Freeman S’s October 2010 paper exploring the alpha or beta-naphthyl isomer form of Naphyrone (Drug Testing and Analysis, doi:10.1002/dta.185; PMID 20886463).
- In 2011, Kelleher C, Christie R, Lalor K, and others published “An overview of new psychoactive substances and the outlets supplying them,” providing insights into emerging psychoactive substances in Ireland (PDF).