As of my last knowledge update in September 2021, MMDA, 3-Methoxy-4,5-methylenedioxyamphetamine, was a relatively obscure designer drug and research chemical. Please note that the market situation may have evolved since then.
The market for MMDA was niche, with a limited online presence. A handful of vendors and sellers, primarily catering to the research chemical community, offered MMDA for sale through specialized online platforms. These vendors typically marketed it as a research chemical rather than for human consumption to navigate legal restrictions.
MMDA, like other designer drugs, was sought after by researchers interested in exploring its pharmacological effects and potential therapeutic applications. However, its demand was less significant than more well-known substances, and its popularity among recreational users remained relatively low.
Given the rapidly changing legal landscape surrounding designer drugs and research chemicals, the availability and legal status of MMDA could vary from jurisdiction to jurisdiction. It’s essential to emphasize that using, purchasing, and selling such substances should always comply with local laws and regulations.
For the most current information on the market situation of MMDA, it is advisable to consult reliable sources and legal authorities, as the landscape for such compounds is subject to change.
Contents
- 1 Summary
- 2 Psychotherapeutic actions
- 3 Pharmacology
- 4 Legal Status
- 5 FAQ
- 5.1 1. What is MMDA?
- 5.2 2. What are the effects of MMDA?
- 5.3 3. Has MMDA been used in psychotherapy?
- 5.4 4. What is the legal status of MMDA?
- 5.5 5. Is MMDA the same as MDMA?
- 5.6 6. Can MMDA be used for recreational purposes?
- 5.7 7. Is MMDA approved for medical use?
- 5.8 8. What precautions should be taken when using or considering MMDA?
- 5.9 9. Are there any known risks or side effects associated with MMDA use?
- 6 References
Summary
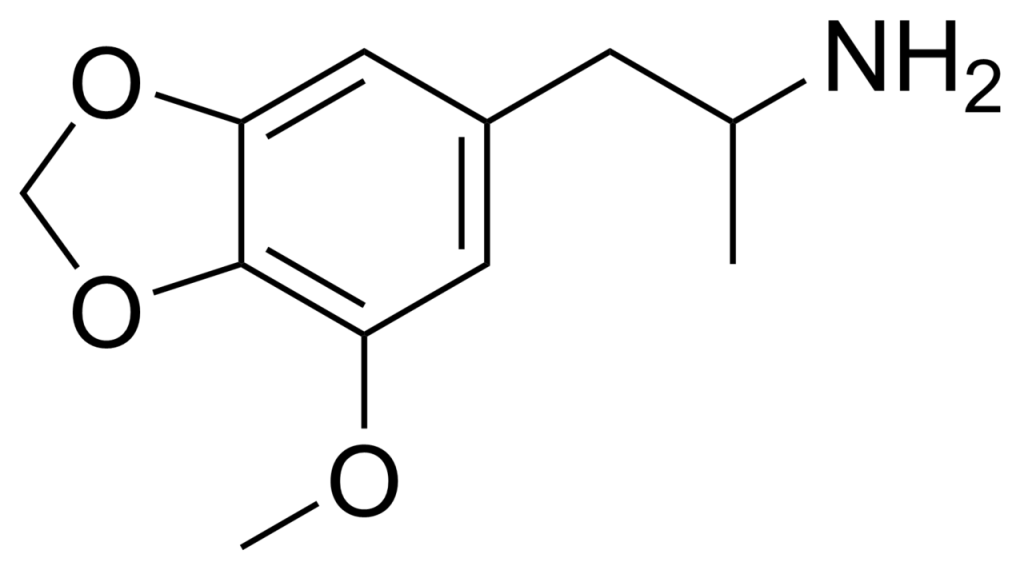
MMDA, scientifically known as 3-methoxy-4,5-methylenedioxyamphetamine or 5-methoxy-MDA, belongs to the amphetamine class of drugs, exhibiting psychedelic and entactogenic properties. It shares chemical similarities with lophophine, MDA, and MDMA.
Alexander Shulgin initially documented the compound MMDA in his influential book, PiHKAL. According to Shulgin, MMDA’s recommended dosage typically falls within 100 to 250 milligrams. Upon oral ingestion, the first effects of MMDA manifest within 30 to 60 minutes.
MMDA induces euphoria, affectionate warmth, and reduced sensations such as anxiety and loneliness. Users also commonly report experiencing closed-eye visuals, drowsiness, muscle relaxation, and distortion of time perception. Some side effects associated with MMDA use encompass moderate pupil dilation (mydriasis), dizziness, sensations of temperature changes (heat or cold), and trembling.
The visual imagery induced by MMDA is often described as realistic and frequently relates to familiar everyday perceptions of people, landscapes, or objects. The effects of MMDA typically peak during the first hour after initial administration, gradually diminishing during the second hour and generally subsiding entirely by the end of the fifth hour.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 13674-05-0 |
|---|---|
| PubChem CID | 26175 |
| ChemSpider | 24386 |
| UNII | RG7FY73ZAA |
| Chemical and physical data | |
| Formula | C11H15NO3 |
| Molar mass | 209.245 g·mol−1 |
Psychotherapeutic actions
In his 1973 publication titled “The Healing Journey,” Claudio Naranjo delved into the psychotherapeutic possibilities MMDA offers. Similar to his observations with MDA, Naranjo noted that MMDA could enhance interpersonal communication and proposed its potential utility within psychotherapy. However, it’s worth noting that as of 2005, MMDA had yet to receive official approval for any human applications on a global scale.
Pharmacology
MMDA has been shown to act as a non-neurotoxic serotonin-releasing agent with no effects on dopamine release and probably norepinephrine release as well and as a 5-HT2A receptor agonist. The latter property is responsible for its psychedelic effects. In contrast, the former mediates its mood-lifting and empathogenic effects.
Legal Status
United States:
In the United States, MMDA is categorized as a Schedule I substance, a classification that is consistent with its controlled status in various regions across the globe. It’s important to emphasize that MMDA remains illegal; however, its legal status differs from that of MDMA, warranting further clarification.
International:
On an international scale, MMDA is classified as a Schedule I drug under the Convention on Psychotropic Substances.
Australia:
MMDA is designated as a Schedule 9 prohibited substance in Australia according to the Poisons Standard (October 2015). This classification signifies that the substance holds the potential for abuse or misuse, and its manufacture, possession, sale, or use is generally prohibited by law. Exceptions may apply when necessary for medical or scientific research or analytical, educational, or training purposes, subject to approval from Commonwealth and State or Territory Health Authorities.
FAQ
1. What is MMDA?
- MMDA, or 3-methoxy-4,5-methylenedioxyamphetamine, is a psychoactive substance classified as an amphetamine. It shares chemical similarities with compounds like MDA and MDMA and is known for its psychedelic and entactogenic properties.
2. What are the effects of MMDA?
- MMDA can induce euphoria and warmth and reduce feelings of anxiety and loneliness. Users may also experience closed-eye visuals, altered time perception, muscle relaxation, and drowsiness. Visual effects are often described as realistic and relatable to everyday experiences.
3. Has MMDA been used in psychotherapy?
- Yes, early explorations by figures like Claudio Naranjo suggested that MMDA could facilitate communication and have potential applications in psychotherapy, similar to MDA. However, these uses have yet to be widely accepted or approved.
4. What is the legal status of MMDA?
- The legal status of MMDA varies by country. It is classified as a Schedule I substance in the United States, making it illegal. Internationally, it is often controlled under drug conventions.
5. Is MMDA the same as MDMA?
- No, MMDA is not the same as MDMA (Ecstasy). While they share some chemical similarities and psychoactive effects, they are distinct substances with different effects and legal classifications.
6. Can MMDA be used for recreational purposes?
- The use of MMDA for recreational purposes is not recommended due to its legal status and potential risks. Additionally, the substance’s effects can vary widely between individuals.
7. Is MMDA approved for medical use?
- As my last knowledge update in September 2021, MMDA was not approved for medical applications in most countries. It is primarily considered a research chemical.
8. What precautions should be taken when using or considering MMDA?
- It is essential to be aware of the legal status of MMDA in your country and to prioritize safety if considering its use. Due to limited research on its safety and effects, caution is advised, and it should only be used under appropriate medical supervision.
9. Are there any known risks or side effects associated with MMDA use?
- Side effects may include mydriasis (pupil dilation), dizziness, sensations of temperature changes, and trembling. The safety profile of MMDA is not well-documented, and there may be unknown risks associated with its use.
References
- Anvisa (2023-07-24). “RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” [Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- McKenna DJ, Guan XM, Shulgin AT (March 1991). “3,4-Methylenedioxyamphetamine (MDA) analogues exhibit differential effects on the synaptosomal release of 3H-dopamine and 3H-5-hydroxytryptamine”. Pharmacology Biochemistry and Behavior. 38 (3): 505–12. doi:10.1016/0091-3057(91)90005-M. PMID 1829838. S2CID 2740262.
- Zhang Z, An L, Hu W, Xiang Y (April 2007). “3D-QSAR study of hallucinogenic phenylalkylamines by using CoMFA approach”. Journal of Computer-aided Molecular Design. 21 (4): 145–53. Bibcode:2007JCAMD..21..145Z. doi:10.1007/s10822-006-9090-y. PMID 17203365. S2CID 25343432.
- “Archived copy” (PDF). Archived from the original (PDF) on 2005-12-05. Retrieved 2005-11-19.
- Poisons Standard October 2015: Link to the document