Contents
- 1 Summary
- 2 Legality
- 3 FAQ
- 3.1 1. What is 4′-Methyl-α-pyrrolidinohexiophenone (MPHP)?
- 3.2 2. How is MPHP related to pyrovalerone?
- 3.3 3. What are the key features of the pyrrolidinophenone series, including MPHP?
- 3.4 4. What health concerns are associated with MPHP use?
- 3.5 5. How is MPHP regulated in the United States?
- 3.6 6. What is the regulatory status of MPHP in Sweden?
- 4 References
Summary
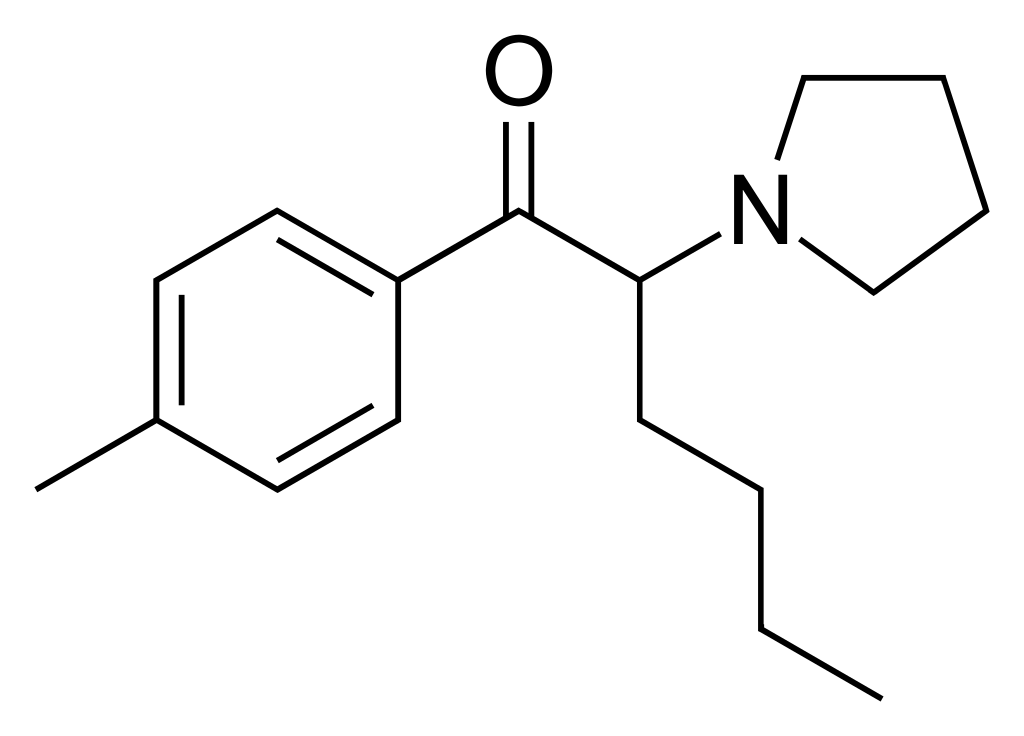
N-Methyl-α-pyrrolidinohexiophenone, often referred to as MPHP, is a stimulant compound recognized as a novel designer drug. It shares a close chemical relationship with pyrovalerone, essentially an extended homologue of the latter. Within the pyrrolidinophenone series, MPHP maintains stimulant properties as long as the positions of the aryl, ketone, and pyrrolidinyl groups remain consistent. At the same time, the alkyl backbone can vary from three to as many as seven carbons. The pentyl or isohexyl backbone exhibits the highest potency, and the aromatic ring can tolerate various substituents.
In 2010, a team of researchers from the Institute of Forensic Medicine at University Hospital Jena, Germany, concluded that using MPHP could lead to severe poisoning, resulting in toxic liver damage and rhabdomyolysis, a serious medical condition.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 34138-58-4 |
|---|---|
| PubChem CID | 6423085 |
| ChemSpider | 4928584 |
| UNII | Y8D4922F5K |
| Chemical and physical data | |
| Formula | C17H25NO |
| Molar mass | 259.393 g·mol−1 |
Legality
In the United States, MPHP is categorized as a Schedule I Controlled Substance, indicating its legal classification with stringent restrictions. Furthermore, on June 1, 2015, Sweden’s public health agency recommended classifying MPHP as a narcotic within its regulatory framework.
FAQ
1. What is 4′-Methyl-α-pyrrolidinohexiophenone (MPHP)?
- 4′-Methyl-α-pyrrolidinohexiophenone, commonly called MPHP, is a stimulant compound often associated with designer drugs.
- MPHP is closely related to pyrovalerone and is an extended homologue of pyrovalerone. It shares chemical similarities with pyrovalerone but has variations in its structure.
3. What are the key features of the pyrrolidinophenone series, including MPHP?
- In the pyrrolidinophenone series, stimulant activity is maintained when the positions of the aryl, ketone, and pyrrolidinyl groups remain constant. The alkyl backbone can vary from three to as many as seven carbons, with the pentyl or isohexyl backbone typically exhibiting the highest potency. Various substituents on the aromatic ring are also tolerated.
4. What health concerns are associated with MPHP use?
- Research conducted in 2010 by a group of researchers in Germany suggested that MPHP use can lead to severe poisoning, including toxic liver damage and rhabdomyolysis, a painful medical condition.
5. How is MPHP regulated in the United States?
- MPHP is classified as a Schedule I Controlled Substance in the United States, signifying its legal status with strict regulatory controls.
6. What is the regulatory status of MPHP in Sweden?
- In Sweden, the public health agency recommended classifying MPHP as a narcotic on June 1, 2015, indicating its legal restrictions within the country’s regulatory framework.
References
- In June 2003, Springer D, Peters FT, Fritschi G, and Maurer HH conducted research on the “New designer drug 4′-methyl-alpha-pyrrolidinohexanophenone.” Their study focused on its metabolism and toxicological detection in urine using gas chromatography-mass spectrometry. This work was published in the “Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences,” Volume 789 (1), pages 79–91, with a DOI of 10.1016/S1570-0232(03)00043-6 and a PMID of 12726846.
- In May 2009, Peters FT, Dragan CA, Kauffels A, Schwaninger AE, Zapp J, Bureik M, and Maurer HH explored the biotechnological synthesis of the designer drug metabolite 4′-hydroxymethyl-alpha-pyrrolidinohexanophenone. They achieved this through fission yeast heterologously expressing human cytochrome P450 2D6, providing a versatile alternative to multistep chemical synthesis. This research was featured in the “Journal of Analytical Toxicology,” Volume 33 (4), pages 190–7, with a DOI of 10.1093/jat/33.4.190 and a PMID of 19470220.
- Sauer C, Hoffmann K, Schimmel U, and Peters FT, in May 2011, investigated “Acute poisoning involving the pyrrolidinophenone-type designer drug 4′-methyl-alpha-pyrrolidinohexanophenone (MPHP).” Their findings were published in “Forensic Science International,” Volume 208 (1–3), pages e20-5, with a DOI of 10.1016/j.forsciint.2011.02.026 and a PMID of 21444164.
- The “GB patent 1149366” relates to “α-substituted-ketones and processes for their preparation.” This patent likely addresses aspects of the synthesis or preparation of related compounds.
- Meltzer PC, Butler D, Deschamps JR, and Madras BK, in February 2006, explored “1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues.” They investigated this promising class of monoamine uptake inhibitors in the “Journal of Medicinal Chemistry,” Volume 49 (4), pages 1420–32, with a DOI of 10.1021/jm050797a and a PMID of 16480278.
- The reference by Sauer C, Hoffmann K, Schimmel U, and Peters FT in May 2011 on “Acute poisoning involving the pyrrolidinophenone-type designer drug 4′-methyl-alpha-pyrrolidinohexanophenone (MPHP)” has been included previously in question 3.
- Information from the “Drug Enforcement Administration” discusses the temporary placement of substances, including MPHP, in Schedule I of Controlled Substances. This document highlights regulatory measures in the United States and is archived as of April 30, 2021.
- The reference related to “23 nya ämnen kan klassas som narkotika eller hälsofarlig vara” pertains to potential regulatory changes in Sweden, suggesting the classification of certain substances as narcotics or hazardous items. This information was retrieved on June 29, 2015.