Contents
Summary
Desomorphine, also known as Dihydrodesoxymorphine, belongs to the morphinan chemical class and exhibits various effects, including analgesia, muscle relaxation, sedation, and euphoria upon administration. This substance is a structural analog of morphine and is a key component in the drug mixture referred to as Krokodil (also known as Crocodile, Krok, or Croc).
Initially developed by Roche in the 1930s, desomorphine was introduced for medical use in Switzerland under the trade name Permonid. It was noted for its rapid onset of action, short duration of effect, relatively low incidence of nausea, and analgesic potency surpassing that of morphine by 8 to 10 times. However, concerns arose regarding its potential for dependence and abuse, ultimately leading to its discontinuation in clinical practice.
Desomorphine gained notoriety in the Russian drug scene around 2003 when it became known as Krokodil. The name “Krokodil” references the scaly, green-black skin discoloration frequently observed in its users. Its popularity can be attributed to the widespread availability of inexpensive over-the-counter codeine tablets and a straightforward production process that can be carried out in makeshift laboratories, utilizing ingredients such as iodine, red phosphorus, paint thinner, and hydrochloric acid.
The use of Krokodil is associated with immediate damage to blood vessels, muscles, and bones, potentially leading to multiple organ failure. It’s important to note that these severe complications are primarily caused by the toxic byproducts generated during the homemade production process rather than the desomorphine itself.
It’s worth mentioning that the scientific literature on Krokodil is limited, and much of the information available is based on media reports. While a few unverified cases of Krokodil use have been reported in countries like Germany and the United States, as of 2018, confirmed instances of Krokodil use are primarily documented in Russia.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 427-00-9 |
|---|---|
| PubChem CID | 5362456 |
| ChemSpider | 4515044 |
| UNII | 7OP86J5E33 |
| KEGG | D12670 |
| ChEMBL | ChEMBL2106274 |
| CompTox Dashboard (EPA) | DTXSID10195390 |
| ECHA InfoCard | 100.006.406 |
| Chemical and physical data | |
| Formula | C17H21NO2 |
| Molar mass | 271.360 g·mol−1 |
History and culture
Desomorphine was initially synthesized and patented in the United States back in 1932. At the time, it was developed with the aim of providing an alternative to morphine that had better tolerance and addiction profiles while also improving the side effect profile. However, desomorphine did not meet these expectations and was found to have a greater potential for dependence compared to morphine.
In Switzerland, desomorphine, known by its trade name Permonid, was introduced to the market in 1940 by the pharmaceutical company Hoffman-La Roche. It gained popularity, particularly for managing postoperative pain, thanks to its rapid onset of action and reduced tendency to cause respiratory depression and nausea. Toward the end of 1952, Permonid was removed from the market. Interestingly, production continued in Switzerland until 1981 due to the unique analgesic requirements of a single patient in Bern who suffered from a rare medical condition.
In Russia, Krokodil is considered an inexpensive and highly addictive substitute for heroin. Its name, “Krokodil,” is derived from the Russian word for crocodile and alludes to the scaly, green-black skin discoloration frequently observed in its users. The production of Krokodil involves synthesizing desomorphine from codeine and mixing it with readily available, low-cost additives. These additives may include hydrochloric acid, red phosphorus (obtained from matchbook striking surfaces), iodine, gasoline, and paint thinner. These components are believed to be responsible for the severe skin and systemic effects associated with Krokodil. The production process bears resemblance to the methods used for street methamphetamine production.
Since 2003, the prevalence of Krokodil use in Russia has been rapidly increasing, likely due to its affordability and its high potential for dependence.
Chemistry
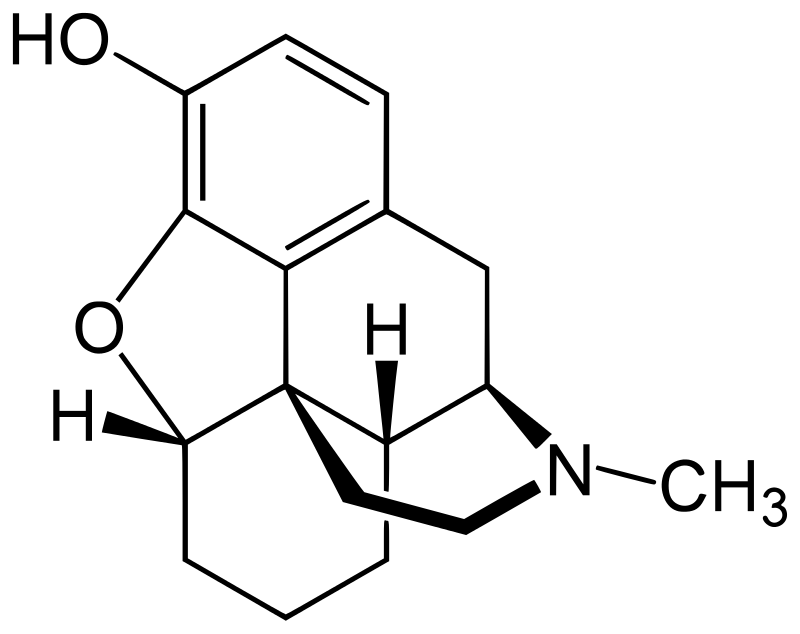
Desomorphine, classified as a benzylisoquinoline alkaloid, belongs to the morphinan class of opioids. Compounds like desomorphine within this class share a common polycyclic structure composed of three benzene rings arranged in a zig-zag pattern known as phenanthrene. An additional nitrogen-containing ring is fused to the phenanthrene structure at positions R9 and R13, with the nitrogen atom positioned at R17 within this combined structure. This distinctive arrangement is referred to as morphinan.
Desomorphine, like other morphinans, features an ether bridge that links two of its rings, specifically connecting R4 and R5 through an oxygen group. It also possesses a hydroxy group (OH-) attached to R3 and a methyl group located on the nitrogen atom at R17. Notable chemical distinctions from morphine include the absence of a secondary hydroxy group at R6 and the presence of a saturated double bond.
Pharmacology
Desomorphine can be synthesized using codeine and iodine sourced from over-the-counter medications, as well as red phosphorus obtained from match strikers following a process akin to the production of methamphetamine using pseudoephedrine. Similar to methamphetamine, desomorphine produced in this manner often contains impurities. In Russia, the street name for homemade desomorphine is “krokodil” (Russian: крокодил, crocodile), possibly derived from the chemical name of the precursor α-chlorocodide or the resemblance of the skin damage caused by the drug to a crocodile’s leather.
Desomorphine is classified as a morphine analog in which the 6-hydroxyl group and the 7,8 double bond have been reduced. The conventional synthesis of desomorphine commences with α-chlorocodide, which is obtained through the reaction of thionyl chloride with codeine. Catalytic reduction of α-chlorocodide yields dihydrodesoxycodeine, which, upon demethylation, produces desomorphine.
As with other opiates, desomorphine exerts its effects by binding to and activating the μ-opioid receptor as an agonist. This mechanism operates by opioids mimicking the function of the body’s natural endorphins, which are responsible for pain relief (analgesia), sedation, and feelings of pleasure and well-being. Endorphins are naturally released in response to stimuli such as pain, vigorous physical activity, orgasm, or excitement. This mimicry of natural endorphins underlies the drug’s capacity to induce euphoria, alleviate pain, and provide anxiolytic (anti-anxiety) effects.
Subjective effects
As there is limited availability of anecdotal reports regarding high-purity desomorphine, the following effects are inferred from early clinical observations and deductions based on its relationship with codeine and morphine. Desomorphine is recognized for its potent euphoric opiate analgesia, characterized by rapid onset and a shorter duration that may promote compulsive use.
Please note that the effects listed below are based on the Subjective Effect Index (SEI). This research literature relies on anecdotal user accounts and the personal assessments of PsychonautWiki contributors. Consequently, they should be approached with a degree of skepticism.
It is also essential to recognize that these effects may not consistently manifest predictably. Nevertheless, higher doses are more likely to induce the full range of effects. Additionally, it’s important to understand that elevated doses can elevate the risk of adverse effects, including addiction, severe harm, or even fatality ☠.
Physical:
- Sedation
- Physical Euphoria
- Constipation
- Cough Suppression
- Reduced Libido
- Difficulty Urinating
- Itchiness
- Nausea (Desomorphine may cause less nausea compared to morphine, as suggested by early clinical studies.)
- Pain Relief
- Pupil Constriction
- Respiratory Depression (Desomorphine may induce less respiratory depression than morphine, according to early clinical studies.)
- Skin Flushing
- Appetite Suppression
- Orgasm Suppression
Cognitive:
- Cognitive Euphoria
- Anxiety Suppression
- Compulsive Redosing (Due to its fast onset and shorter duration, this effect may be more pronounced compared to other opiates like morphine.)
- Dream Potentiation
Visual:
- Visual Suppressants
- Double Vision (At high doses, the eyes may lose focus and refocus uncontrollably, resulting in blurriness and double vision that persists regardless of where one tries to focus. This effect can become so intense that reading or driving becomes impossible.)
- Hallucinatory States
- Internal Hallucination (During heavy nodding at high doses, individuals may enter a semi-conscious state and experience hypnagogic imagery, resembling dream-like states. Vague geometric patterns often accompany this.)
Toxicity
Desomorphine, much like most opioids, does not inherently produce many long-term complications aside from dependence and constipation. The detrimental or toxic aspects of desomorphine use are primarily associated with improper administration, overdose, and the use of impure products stemming from substandard black-market production.
Animal studies comparing pure desomorphine to morphine indicate increased toxicity, heightened pain relief, greater sedation, decreased respiration, and increased digestive activity.
Heavy doses of desomorphine can lead to respiratory depression, potentially resulting in fatal or perilous oxygen deprivation (anoxia). This occurs because the drug suppresses the breathing reflex by activating µ-opioid receptors, with the degree of suppression correlating with the consumed dosage.
Additionally, desomorphine may induce nausea and vomiting, which can be particularly hazardous. A significant number of opioid overdose-related deaths occur due to unconscious individuals aspirating vomit. This happens when an unconscious or semi-conscious person lying on their back vomits into their mouth, unknowingly causing suffocation. This risk can be mitigated by ensuring that the individual is positioned on their side with their head tilted downward, preventing airway blockage in case of vomiting while unconscious (a technique known as the recovery position).
Toxicity of “Krokodil”
Illicitly produced desomorphine is typically far from pure and often contains substantial amounts of toxic substances due to suboptimal synthesis processes. Injecting such mixtures can lead to severe damage to the skin, blood vessels, bone, and muscles, sometimes necessitating limb amputation in long-term users.
This damage results from the presence of iodine, phosphorus, and residual solvents, such as gasoline and paint thinner, which are insufficiently removed after synthesis. Strong acids and bases like hydrochloric acid and sodium hydroxide are also used without proper pH measurement of the final solution. Failure to eliminate insoluble fillers and binding agents from codeine tablets used as a starting material, as well as co-administration with pharmaceuticals like tropicamide and tianeptine, are also potential contributors to the observed high toxicity among users.
The frequent occurrence of tissue damage and infections among illicit users earned the drug its notorious nickname, the “flesh-eating drug.” It’s important to emphasize that pure desomorphine itself does not cause this damage. Despite the severe health consequences and short survival times often reported, there are rare cases of “krokodil” users who are more adept at the manufacturing process and have used the drug for extended periods without experiencing the tissue damage associated with the impure “street” product.
Using harm reduction practices is strongly recommended when using this drug.
Tolerance and Addiction Potential
As with other opiate-based painkillers, chronic desomorphine use can lead to significant addiction, resulting in both physical and psychological dependence. Individuals who develop physical dependence may experience withdrawal symptoms if they suddenly discontinue use.
Tolerance to many of desomorphine’s effects, including its therapeutic effects, develops with prolonged use. This necessitates progressively higher doses to achieve the same effects, with tolerance to euphoric effects developing more rapidly. Desomorphine also leads to cross-tolerance with all other opioids, reducing the effectiveness of other opioids following desomorphine consumption.
Overdose
Overdose from desomorphine can result in death, and the time it takes to reach this outcome can vary from several minutes to several hours. Typically, death occurs due to oxygen deprivation resulting from suppressed respiration. Many fatalities attributed to opioid overdoses may be due to interactions with other depressants like alcohol or benzodiazepines. Additionally, opioid-induced nausea and vomiting can lead to death by aspiration of vomit in unconscious individuals.
The risk of fatal opioid overdoses significantly increases after a period of cessation and relapse, largely due to reduced tolerance. To account for this lack of tolerance, it is safer to administer a fraction of the usual dose when relapsing. Moreover, the environment in which opioids are consumed can impact tolerance levels. In a scientific study, rats with a history of heroin administration were significantly more likely to die after receiving their dose in an unfamiliar environment compared to a familiar one.
Opioid overdose is typically treated with an opioid antagonist like naloxone (Narcan), which reverses opioid effects and restores consciousness. However, it may induce withdrawal symptoms. Naloxone has a shorter half-life than most opioids, so it may need to be administered multiple times until the body metabolizes the opioid.
Dangerous Interactions
Warning: Many psychoactive substances, which are reasonably safe when used independently, can become dangerous and even life-threatening when combined with certain other substances. The following list includes some known hazardous interactions (though it may not encompass all of them).
Always conduct independent research (e.g., Google, DuckDuckGo, PubMed) to ensure the safety of combining two or more substances. Some of the interactions listed here are sourced from TripSit.
- Depressants (1,4-Butanediol, 2M2B, alcohol, barbiturates, GHB/GBL, methaqualone, opioids): This combination can result in dangerous or even fatal levels of respiratory depression. These substances potentiate each other’s muscle relaxation and sedation, potentially leading to unexpected loss of consciousness at high doses. Vomiting during unconsciousness and death from suffocation become more likely. If this occurs, individuals should attempt to sleep in the recovery position or have a friend help them get into this position.
Legal status
Germany: Desomorphine falls under BtMG Anlage I in Germany, rendering it unlawful to produce, import, possess, sell, or transfer without the appropriate license.
Russia: Desomorphine is categorized as a Schedule I controlled substance in Russia.
Switzerland: Switzerland designates Desomorphine as a controlled substance explicitly listed under Verzeichnis A. Medicinal applications are allowed.
United States: Desomorphine holds Schedule I status in the United States, meaning it is illegal to manufacture, purchase, possess, or distribute it without a DEA license.
FAQ
- What is Desomorphine?
- Desomorphine is an opioid substance belonging to the morphinan chemical class. It is derived from codeine and has potent analgesic (pain-relieving) properties.
- What are the common street names for Desomorphine?
- Desomorphine is often referred to as “Krokodil” due to the scaly, green-black skin discoloration frequently observed in its users. Other street names include “Crocodile,” “Krok,” or “Croc.”
- Is Desomorphine legal anywhere?
- Desomorphine is typically classified as a controlled substance or illegal drug in many countries, including the United States, Russia, Germany, and Switzerland. It is generally not available for legal use without a prescription.
- Why is Desomorphine called “Krokodil”?
- The street name “Krokodil” is thought to be related to the chemical name of one of its precursors, α-chlorocodide, or the resemblance of the severe skin damage caused by the drug to a crocodile’s leather.
- What are the effects of Desomorphine use?
- Desomorphine use can lead to various effects, including euphoria, sedation, pain relief, and anxiety suppression. However, it can also result in serious health issues and addiction.
- Is Desomorphine safe to use?
- Desomorphine is associated with severe health risks, including addiction, tissue damage, and infections. The impurities often present in illegally produced Desomorphine contribute to its dangerous effects.
- How is Desomorphine produced?
- Desomorphine is typically synthesized from codeine and various additives, including toxic substances like iodine and phosphorus. Illicit production methods often result in impure and dangerous forms of the drug.
- What are the signs of Desomorphine use or abuse?
- Signs of Desomorphine use may include marked changes in behavior, physical appearance, and health. Users may exhibit severe skin damage, dental problems, and deteriorating overall well-being.
- How is Desomorphine addiction treated?
- Desomorphine addiction is typically treated with interventions such as counseling, therapy, and, in some cases, medication-assisted treatment. Medical professionals can guide the most suitable treatment options.
- Is there a safe way to use Desomorphine?
- No, there is no safe way to use Desomorphine recreationally. Due to its high risk of addiction and severe health consequences, it is strongly discouraged.
Please note that Desomorphine is a highly dangerous drug, and this FAQ is intended for informational purposes only. It is essential to seek help and support from healthcare professionals if you or someone you know is struggling with Desomorphine addiction.
References
- Hackenthal E. Desomorphin. Hintergrund. (Desomorphine Background). In: Hagers Handbuch der Pharmazeutischen Praxis (Hager’s compendium of pharmaceutical practice). vol 4, 5th ed. Berlin: NOVOSTI, 1998.
- Sargent, L. J., May, E. L. (November 1970). “Agonists-antagonists derived from desomorphine and metopon”. Journal of Medicinal Chemistry. 13 (6): 1061–1063. doi:10.1021/jm00300a009. ISSN 0022-2623.
- Janssen, P. A. J. (April 1962). “A review of the chemical features associated with strong morphine-like activity”. British Journal of Anaesthesia. 34 (4): 260–268. doi:10.1093/bja/34.4.260. ISSN 0007-0912.
- Bognar R, Makleit S. Neue Methode für die Vorbereitung von dihydro-6-desoxymorphine (A new method for the preparation of dihydro-6-desoxymorphinan). Arzneimittelforschung 1958; 6:323–5.
- Russian News & Information Agency NOVOSTI. Desomorphin. Hintergrund (Desomorphine. Background). Link (accessed December 20, 2011).
- Grund, Jean-Paul C.; Latypov, Alisher; Harris, Magdalena (2013). “Breaking worse: The emergence of krokodil and excessive injuries among people who inject drugs in Eurasia”. International Journal of Drug Policy. 24 (4): 265–274. doi:10.1016/j.drugpo.2013.04.007. ISSN 0955-3959.
- Gahr, Maximilian; Freudenmann, Roland W.; Hiemke, Christoph; Gunst, Ingo M.; Connemann, Bernhard J.; Schönfeldt-Lecuona, Carlos (2012). “Desomorphine Goes “Crocodile””. Journal of Addictive Diseases. 31 (4): 407–412. doi:10.1080/10550887.2012.735570. ISSN 1055-0887.
- Frederick, S. L., Morphine derivative and processes for its preparation.
- Small, L. F., Yuen, K. C., Eilers, L. K. (September 1933). “The Catalytic Hydrogenation of the Halogenomorphides: Dihydrodesoxymorphine-D 1”. Journal of the American Chemical Society. 55 (9): 3863–3870. doi:10.1021/ja01336a073. ISSN 0002-7863.
- Eddy, N. B., Howes, H. A. (1 November 1935). “Studies of Morphine, Codeine and Their Derivatives X. Desoxymorphine-C, Desoxycodeine-C and Their Hydrogenated Derivatives”. Journal of Pharmacology and Experimental Therapeutics. 55 (3): 257–267. ISSN 0022-3565.
- Alves, Emanuele Amorim; Grund, Jean-Paul Cornelis; Afonso, Carlos Manuel; Netto, Annibal Duarte Pereira; Carvalho, Félix; Dinis-Oliveira, Ricardo Jorge (2015). “The harmful chemistry behind krokodil (desomorphine) synthesis and mechanisms of toxicity”. Forensic Science International. 249: 207–213. doi:10.1016/j.forsciint.2015.02.001. ISSN 0379-0738.
- Shelton, Megan; Ramirez-Fort, Marigdalia K.; Lee, Kachiu C.; Ladizinski, Barry (2015). “Krokodil”. JAMA Dermatology. 151 (1): 32. doi:10.1001/jamadermatol.2014.1025. ISSN 2168-6068.
- Savchuk, S. A., Barsegyan, S. S., Barsegyan, I. B., Kolesov, G. M. (April 2008). “Chromatographic study of expert and biological samples containing desomorphine”. Journal of Analytical Chemistry. 63 (4): 361–370. doi:10.1134/S1061934808040096. ISSN 1061-9348.
- Mosettig, E., Cohen, F. L., Small, L. F. (February 1932). “Desoxycodeine Studies. III. The Constitution of the So-Called α-Dihydrodesoxycodeine: Bis-Dihydrodesoxycodeine”. Journal of the American Chemical Society. 54 (2): 793–801. doi:10.1021/ja01341a051. ISSN 0002-7863.
- Merck Manual of Home Health Handbook – 2nd edition, 2003, p. 2097.
- Katselou, M., Papoutsis, I., Nikolaou, P., Spiliopoulou, C., Athanaselis, S. (May 2014). “A “Krokodil” emerges from the murky waters of addiction. Abuse trends of an old drug”. Life Sciences. 102 (2): 81–87. doi:10.1016/j.lfs.2014.03.008. ISSN 0024-3205.
- Haskin, A., Kim, N., Aguh, C. (March 2016). “A new drug with a nasty bite: A case of krokodil-induced skin necrosis in an intravenous drug user”. JAAD Case Reports. 2 (2): 174–176. doi:10.1016/j.jdcr.2016.02.007. ISSN 2352-5126.
- Darke, S., Zador, D. (December 1996). “Fatal heroin ‘overdose’: a review”. Addiction. 91 (12): 1765–1772. doi:10.1046/j.1360-0443.1996.911217652.x. ISSN 0965-2140.
- Why Heroin Relapse Often Ends In Death – Lauren F Friedman (Business Insider) | Link
- Siegel, S., Hinson, R. E., Krank, M. D., McCully, J. (23 April 1982). “Heroin “Overdose” Death: Contribution of Drug-Associated Environmental Cues”. Science. 216 (4544): 436–437. doi:10.1126/science.7200260. ISSN 0036-8075.
- Anlage I BtMG – Einzelnorm
- Постановление Правительства РФ от 01.10.2012 N 1002 (ред. от 09.08.2019)
- “Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien” (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.