Contents
Summary
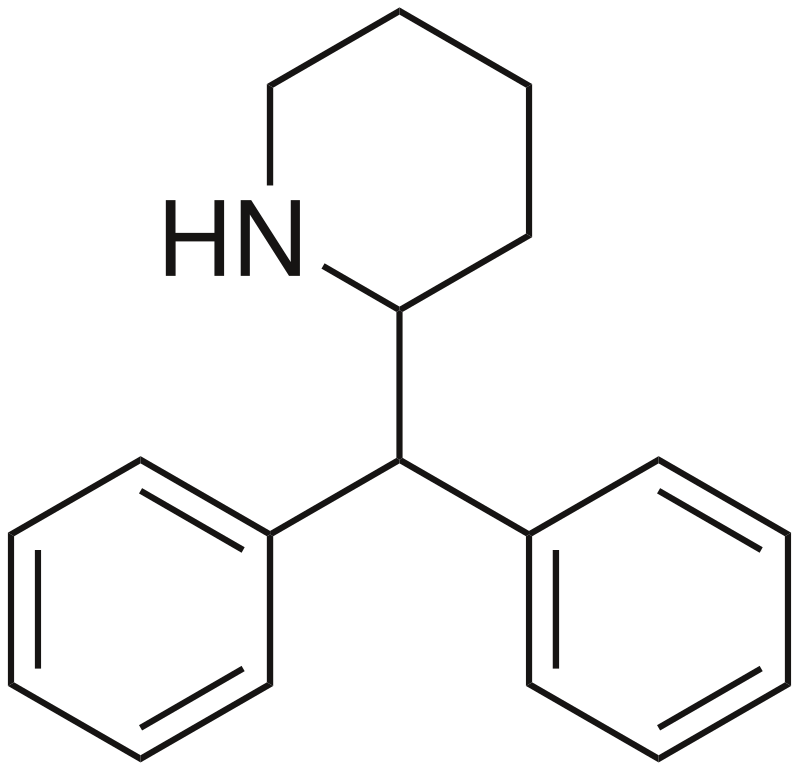
Desoxypipradrol, also known as 2-DPMP, 2-diphenylmethylpiperidine, or Ivory Wave, is a stimulant drug based on the benzylpiperidine structure. It shares a close chemical relationship with methylphenidate and pipradol.
Initially developed by Ciba in the 1950s, desoxypipradrol was explored for various purposes, including the treatment of conditions like narcolepsy and ADHD. However, its development was halted when the same company created methylphenidate, which was deemed superior for ADHD treatment due to its shorter duration of action and more predictable pharmacokinetics. Although desoxypipradrol was researched for alternative applications, such as aiding rapid recovery from anesthesia, its development was discontinued. Nevertheless, a hydroxylated derivative called pipradrol was introduced as a clinical drug for addressing depression, narcolepsy, and cognitive enhancement in cases of organic dementia.
Like methylphenidate and pipradol, desoxypipradrol is believed to function as a norepinephrine-dopamine reuptake inhibitor (NDRI). Among these three piperidines, it’s worth noting that desoxypipradrol possesses the lengthiest elimination half-life. This is attributed to its highly lipophilic nature and a lack of polar functional groups that are typically targeted by metabolic enzymes. Consequently, it exhibits an unusually prolonged duration of action compared to most stimulants. The combination of its extended action and ultra-high potency (starting at 2mg) has given this compound a reputation for being extremely hazardous when abused or mishandled.
Desoxypipradrol is seldom if ever, encountered on the illicit market. Instead, it is generally distributed as a gray-market research chemical by online vendors.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 519-74-4 5807-81-8 (HCl) |
|---|---|
| PubChem CID | 160506 |
| ChemSpider | 141045 |
| UNII | 49UNK1BV8T |
| CompTox Dashboard (EPA) | DTXSID90897502 |
| ECHA InfoCard | 100.007.525 |
| Chemical and physical data | |
| Formula | C18H21N |
| Molar mass | 251.373 g·mol−1 |
Chemistry
Desoxypipradrol belongs to the synthetic stimulant group known as substituted benzylpiperidines. Its structure features a covered phenethylamine framework with an added phenyl ring connected to the Rβ position. The terminal amino group within the phenethylamine chain is integrated into a piperidine ring. Due to its absence of polar functional groups, it possesses high lipophilicity, granting it an exceptionally prolonged, multi-day duration of action, a characteristic rarely seen in stimulant drugs, except for MDPV.
Pharmacology
Desoxypipradrol functions primarily as a norepinephrine-dopamine reuptake inhibitor (NDRI). However, it stands out due to its remarkable potency, prolonged duration of action, and unpredictable dose-response characteristics compared to typical stimulant drugs. These distinctive properties are likely attributed to its exceptionally high lipophilicity and extended elimination half-life. These factors may elevate the risk of inducing stimulant psychosis when misused, a condition for which it has been observed to have a shallow threshold in humans.
Subjective effects
Desoxypipradrol is known for its exceptional potency, prolonged duration, and unpredictable response compared to typical stimulant drugs. However, users have reported a tendency to compulsively re-dose this substance due to its subtle stimulation, which can lead to dangerous levels of consumption, potentially resulting in overdose, hospitalization, or even fatalities. Anyone considering experimenting with this compound should exercise extreme caution, avoid redosing, and utilize proper harm-reduction techniques, including volumetric dosing.
Disclaimer: The effects listed below are based on anecdotal user reports and the subjective experiences reported in the Subjective Effect Index (SEI). These effects should be approached with skepticism, as they may not occur predictably or reliably. Higher doses are more likely to induce a broader range of products, and excessive amounts can lead to addiction, severe harm, or even death ☠.
Physical:
- Stimulation: Desoxypipradrol is generally regarded as energetic and stimulating. Its motivation is more potent than modafinil and caffeine but subtler than amphetamine or methamphetamine. Lower to moderate doses promote productivity, while higher doses may encourage physical activities like dancing, socializing, running, or cleaning. Notably, its stimulation feels clean and lucid, with manic undertones.
- Spontaneous Physical Sensations
- Increased Heart Rate: Initial reports suggest that desoxypipradrol’s impact on heart rate and blood pressure is relatively weak.
- Dehydration
- Appetite Suppression
- Increased Perspiration: Compared to classical stimulants, desoxypipradrol appears to involve less peripheral nervous system stimulation, resulting in a “cleaner” or more lucid experience with fewer bodily effects like sweating or cramping.
- Teeth Grinding: Unlike amphetamines and other stimulants, teeth grinding is absent mainly due to the subtleness of desoxypipradrol’s stimulation.
- Headaches
- Restless Leg Syndrome
- Vasoconstriction: Minimal compared to many other stimulants and stimulating drugs.
- Stamina Enhancement
- Bodily Control Enhancement
Visual:
- Acuity Enhancement: Occurs unpredictably, typically at moderate to high doses, but can occasionally happen at lower doses. When it does, the effect tends to be subtler than other visual acuity-enhancing stimulants like MDMA or methamphetamine.
- Brightness Alteration: Unpredictable and usually seen at moderate to high doses, with occasional occurrences at lower doses. The effect, when present, tends to be subtler than other visual brightness-altering stimulants like MDMA or methamphetamine.
- Drifting: Occurs unpredictably, typically at moderate to high doses, but may also happen at lower doses. The effect, when it occurs, is subtler than other vision-altering stimulants like MDMA or methamphetamine.
Cognitive:
- Euphoria: Compared to other stimulants like amphetamine, the euphoria induced by desoxypipradrol feels less forced and more unintrusive.
- Focus Enhancement: Most effective at low to moderate dosages, as higher doses tend to impair concentration.
- Wakefulness
- Memory Enhancement
- Novelty Enhancement
- Thought Acceleration
- Thought Organization
- Thought Connectivity
- Analysis Enhancement
- Motivation Enhancement
- Immersion Enhancement
- Stamina Enhancement
- Ego Inflation
- Mania
- Emotion Suppression: Typically seen at medium to high doses, similar to the emotion suppression exhibited by methylphenidate use.
- Information Processing Suppression
- Language Suppression
- Anxiety Suppression: Occasionally observed, usually at lower doses, and tends to decrease as the dose increases.
- Thought Deceleration: Typically seen at higher doses but can occasionally occur at lower doses unpredictably. At lower to medium quantities, though acceleration is more common.
- Thought Disorganization: Typically seen at higher doses but can occasionally happen at lower doses unpredictably. At lower to medium quantities, thought organization is more common.
- Compulsive Redosing
- Delusion
- Paranoia
- Delirium
- Depersonalization
- Derealization
- Psychosis
After:
- Anxiety
- Cognitive Fatigue
- Depression
- Irritability
- Motivation Suppression
- Thought Deceleration
- Wakefulness
Toxicity
Desoxypipradrol can be quantified in blood, plasma, or urine using liquid chromatography-mass spectrometry to confirm poisoning in hospitalized patients or as evidence in medicolegal death investigations. Recreational users typically exhibit blood or plasma concentrations ranging from 10 to 50 μg/L, while intoxicated individuals may have levels exceeding 100 μg/L. In cases of acute overdose, victims may show concentrations surpassing 600 μg/L.
It is highly advisable to practice harm reduction when using this substance.
Tolerance and Addiction Potential
Desoxypipradrol can be used consecutively for extended periods, leading to the development of acute tolerance to many of its effects with prolonged and repeated use. Users may find themselves requiring progressively larger doses to achieve the same impact. Tolerance reduction typically takes approximately 3 to 7 days to reach half of the previous level and 1 to 2 weeks to return to baseline (in the absence of further consumption). Desoxypipradrol induces cross-tolerance with all dopaminergic stimulants, diminishing the impact of other stimuli following desoxypipradrol use.
Similar to other stimulants, chronic use of desoxypipradrol can be moderately addictive, carrying a high potential for abuse and the potential for psychological dependence in certain users. When addiction develops, users may experience cravings and withdrawal symptoms upon discontinuation.
User reports indicate that desoxypipradrol poses unique and atypical hazards when misused, especially when administered by eyeballing (without precise measurement) or mishandled without proper caution. Desoxypipradrol, like other stimulants, elevates dopamine levels in the brain, which can result in acute manic psychosis and long-term downregulation of dopamine receptors.
Psychosis
Chronic abuse or single-exposure overdose of desoxypipradrol appears to have a heightened potential to induce psychosis compared to the majority of stimulants. Psychotic symptoms associated with desoxypiprol misuse include auditory hallucinations, visual hallucinations, self-harm urges, severe anxiety, mania, grandiosity, paranoid delusions, confusion, heightened aggression, and irritability.
Dangerous Interactions
Warning: Many psychoactive substances that are reasonably safe when used alone can become difficult or life-threatening when combined with certain other substances. The following list outlines some known dangerous interactions (although it may not encompass all possibilities). Always conduct independent research (e.g., using Google, DuckDuckGo, or PubMed) to ensure the safety of combining two or more substances. Some interactions listed here have been sourced from TripSit.
- 25x-NBOMe & 25x-NBOH: These compounds are highly stimulating and physically demanding. Combining them with desoxypipradrol should be strictly avoided due to the risk of excessive stimulation and heart strain, which may result in increased blood pressure, vasoconstriction, panic attacks, thought loops, seizures, and, in extreme cases, heart failure.
- Alcohol: Mixing alcohol with stimulants can be dangerous due to the risk of accidental over-intoxication. Stimulants can mask alcohol’s depressant effects, making it challenging to gauge intoxication. After the motivation wears off, the depressant effects can lead to blackouts and severe respiratory depression. If combined, limit alcohol consumption carefully.
- DXM: Combinations with DXM should be avoided due to DXM’s inhibiting effects on serotonin and norepinephrine reuptake. This combination carries an increased risk of panic attacks and hypertensive crisis, or serotonin syndrome when combined with serotonin releasers (e.g., MDMA, methylone, and mephedrone). Monitor blood pressure carefully and avoid strenuous physical activity.
- MDMA: The neurotoxic effects of MDMA may be intensified when combined with other stimulants, potentially resulting in excessive blood pressure and heart strain (cardiotoxicity).
- MXE: Some reports suggest that combining MXE with desoxypipradrol may dangerously increase blood pressure and the risk of mania and psychosis.
- Dissociatives: Both classes of substances carry a risk of delusions, mania, and psychosis, and this risk may be magnified when combined.
- Stimulants: Combining desoxypipradrol with other motivations, such as cocaine, can lead to a dangerous increase in heart rate and blood pressure.
- Tramadol: Tramadol is known to lower the seizure threshold, and combining it with stimulants may further increase this risk.
- MAOIs: This combination may elevate neurotransmitter levels, including dopamine, to dangerous or even fatal levels. Examples of substances in this category include Syrian rue, Banisteriopsis caapi, and certain antidepressants.
- Cocaine: Combining cocaine with desoxypipradrol may put additional strain on the heart.
Always exercise extreme caution and consult available resources before combining substances to ensure safety.
Legal status
China: As of October 2015, 2-DPMP is classified as a controlled substance in China.
Germany: Desoxypipradrol falls under Anlage II BtMG (Narcotics Act, Schedule II) as of December 13, 2014. It is prohibited to manufacture, possess, import, export, purchase, sell, obtain, or distribute it without a license.
Russia: Deoxypipradol is listed as a Schedule I prohibited substance under the name (Пиперидин-2-ил)дифенилметан.
Switzerland: Desoxypipradol is not regulated under Buchstabe A, B, C, or D, and thus, it may be considered legal.
United Kingdom: On November 4, 2010, the UK Home Office imposed a ban on the importation of 2-DPMP following a recommendation from the ACMD (Advisory Council on the Misuse of Drugs). Before the import ban, desoxypipradrol was sold as a ‘legal high’ in various products. Its use led to several Emergency Department visits, prompting a review by the ACMD. In their report, the ACMD stated that “there are serious harms associated with 2-DPMP… typically prolonged agitation (lasting up to 5 days after drug use, which is sometimes severe and requires physical restraint), paranoia, hallucinations, and myoclonus (muscle spasms/twitches).” Although 2-DPMP was initially scheduled to become a class B drug on March 28, 2012, this decision was reconsidered. The bill was rewritten, and 2-DPMP was eventually classified as a class B drug and placed in Schedule I on June 13, 2012. No recorded deaths were attributed to the drug between the banning of its import and the prohibition of its possession. “Esters and ethers of pipradrol” were also controlled under the same amendment as class C drugs.
United States: Desoxypipradrol remains unscheduled in the United States, making it legal to possess and import. However, it is worth noting that it is an analog of pipradol, which is categorized as a Schedule IV Controlled Substance.
FAQ
1. What is Desoxypipradrol?
Desoxypipradrol, also known as 2-DPMP, is a synthetic stimulant drug. It is classified as a substituted benzylpiperidine and is chemically related to substances like methylphenidate and pipradol.
2. How is Desoxypipradrol typically used?
Desoxypipradrol is primarily used recreationally, although it is not commonly found on the streets. Instead, it is often sold as a research chemical through online vendors. It is typically consumed orally, but users should exercise extreme caution due to its potency and potential for harm.
3. What are the effects of Desoxypipradrol?
Desoxypipradrol is known for its stimulant effects, including increased energy levels, alertness, and enhanced focus. Lower to moderate doses can promote productivity, while higher doses may lead to physical activities such as dancing or socializing. It can also have side effects like increased heart rate, dehydration, and appetite suppression.
4. Is Desoxypipradrol safe to use?
Desoxypipradrol is associated with several risks, including its high potency, unpredictable dose-response, and potential for addiction. Its extended duration of action, combined with its power, makes it particularly risky when misused. Users are advised to exercise extreme caution and harm-reduction practices if considering experimentation.
5. Can Desoxypipradrol lead to addiction?
Yes, like many stimulants, Desoxypipradrol can be addictive and has a high potential for abuse. Chronic use can result in psychological dependence, cravings, and withdrawal effects if usage is suddenly stopped.
6. What is the legal status of Desoxypipradrol in various countries?
The legal status of Desoxypipradrol varies from one country to another. For example, it is classified as a controlled substance in China and Germany. In the United Kingdom, it was banned due to safety concerns. However, in the United States, it remains unscheduled, making it legal to possess and import.
7. Are there any dangerous interactions with Desoxypipradrol and other substances?
Yes, there are potentially dangerous interactions with Desoxypipradrol when combined with certain substances. It’s essential to conduct thorough research to ensure the safety of any substance combinations. Interactions with alcohol, other stimulants, and specific medications can be hazardous.
8. How is Desoxypipradrol’s toxicity measured or detected?
Desoxypipradrol can be quantitated in blood, plasma, or urine by using liquid chromatography-mass spectrometry. This method is used to confirm poisoning in hospitalized patients or to provide evidence in medicolegal death investigations.
9. What should I do if I suspect someone has overdosed on Desoxypipradrol?
If you suspect someone has overdosed on Desoxypipradrol, seek immediate medical attention. Overdose symptoms may include extreme agitation, hallucinations, seizures, and potentially life-threatening complications.
10. Where can I find more information about Desoxypipradrol?
For additional information about Desoxypipradrol, it’s recommended to consult reliable sources, such as government health agencies, medical professionals, or harm reduction organizations. Always prioritize safety and responsible decision-making when considering the use of substances like Desoxypipradrol.
References
- Ferris, R. M., Tang, F. L. (September 1979). “Comparison of the effects of the isomers of amphetamine, methylphenidate, and deoxypipradrol on the uptake of l-[3H]norepinephrine and [3H]dopamine by synaptic vesicles from rat whole brain, striatum, and hypothalamus”. The Journal of Pharmacology and Experimental Therapeutics. 210 (3): 422–428. ISSN 0022-3565.
- Baselt, R. C. (2014). Disposition of toxic drugs and chemicals in man (Tenth edition ed.). Biomedical Publications. ISBN 9780962652394.
- Talaie, H.; Panahandeh, R.; Fayaznouri, M. R.; Asadi, Z.; Abdollahi, M. (2009). “Dose-independent occurrence of seizure with tramadol”. Journal of Medical Toxicology. 5 (2): 63–67. doi:10.1007/BF03161089. eISSN 1937-6995. ISSN 1556-9039. OCLC 163567183.
- Gillman, P. K. (2005). “Monoamine oxidase inhibitors, opioid analgesics, and serotonin toxicity”. British Journal of Anaesthesia. 95 (4): 434–441. doi:10.1093/bja/aei210 Freely accessible. eISSN 1471-6771. ISSN 0007-0912. OCLC 01537271. PMID 16051647.
- “关于印发《非药用类麻醉药品和精神药品列管办法》的通知” (in Chinese). China Food and Drug Administration. September 27, 2015. Retrieved October 1, 2015.
- “Anlage II BtMG” (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 28, 2019.
- “Achtundzwanzigste Verordnung zur Änderung betäubungsmittelrechtlicher Vorschriften” (PDF) (in German). Bundesanzeiger Verlag. Retrieved December 25, 2019.
- “§ 29 BtMG” (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 25, 2019.
- Resolution of the Government of the Russian Federation | [Link to the resolution]
- “Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien” (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
- Import ban on psychoactive drug
- “ACMD advice on ‘Ivory Wave'” (PDF). UK Home Office. January 27, 2012. Retrieved March 11, 2012.
- “The Misuse of Drugs Act 1971 (Amendment) Order 2012” (PDF). UK Home Office. January 27, 2012. Retrieved March 11, 2012.
- “Government accepts ACMD’s advice to schedule D2PM, 2-DPMP, and phenzepam” (PDF). UK Home Office. January 27, 2012. Retrieved March 11, 2012.
- “ACMD letter on further advice on the classification of two steroidal substances – February 2012” (PDF). UK Home Office. February 14, 2012. Retrieved March 18, 2012.
- “Draft Misuse of Drugs Act 1971 (Amendment) Order 2012”. UK Home Office. April 23, 2012. Retrieved May 4, 2012.
- “A Change to the Misuse of Drugs Act 1971: control of pipradrol-related compounds and phenazepam”. UK Home Office. June 7, 2012. Retrieved July 30, 2012.