Summary
Mirtazapine, marketed under the trade name Remeron and others, belongs to the piperazinoazepine class of antidepressant drugs. At elevated doses, it has been reported to exhibit atypical psychedelic and soothing properties. It is categorized as a noradrenergic and specific serotonergic antidepressant (NaSSA).
Initially developed in the Netherlands, mirtazapine entered the United States pharmaceutical market in 1996. Following the expiration of its patent in 2004, generic versions became readily available. Mirtazapine is primarily employed in the management of major depressive disorder and various mood disorders. Additionally, it finds off-label use in addressing generalized anxiety disorder, social anxiety disorder, obsessive-compulsive disorder, panic disorder, post-traumatic stress disorder, diminished appetite, insomnia, nausea/vomiting, itching, and headaches, including migraines.
Higher doses of mirtazapine, surpassing the recommended prescription dosage, are known to induce a unique blend of psychedelic and sedative effects. Users have reported experiencing sedation, as well as mild to moderate open and closed-eye visuals, enhanced conceptual thinking, and feelings of euphoria. Notably, mirtazapine exhibits a peculiar paradox in its sedative effects, with anecdotal accounts and studies suggesting that its sedation diminishes as the dose escalates. For instance, 7.5mg may induce more pronounced sedation than 15mg. One hypothesis proposes minor stimulant effects that may override its soothing properties.
The toxicity and potential health risks associated with recreational mirtazapine use remain largely unknown. Therefore, it is strongly recommended to exercise harm reduction practices when considering the use of this substance.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 85650-52-8 |
|---|---|
| PubChem CID | 4205 |
| IUPHAR/BPS | 7241 |
| DrugBank | DB00370 |
| ChemSpider | 4060 |
| UNII | A051Q2099Q |
| KEGG | D00563 |
| ChEBI | CHEBI:6950 |
| ChEMBL | ChEMBL654 |
| CompTox Dashboard (EPA) | DTXSID0023325 |
| ECHA InfoCard | 100.080.027 |
| Chemical and physical data | |
| Formula | C17H19N3 |
| Molar mass | 265.360 g·mol−1 |
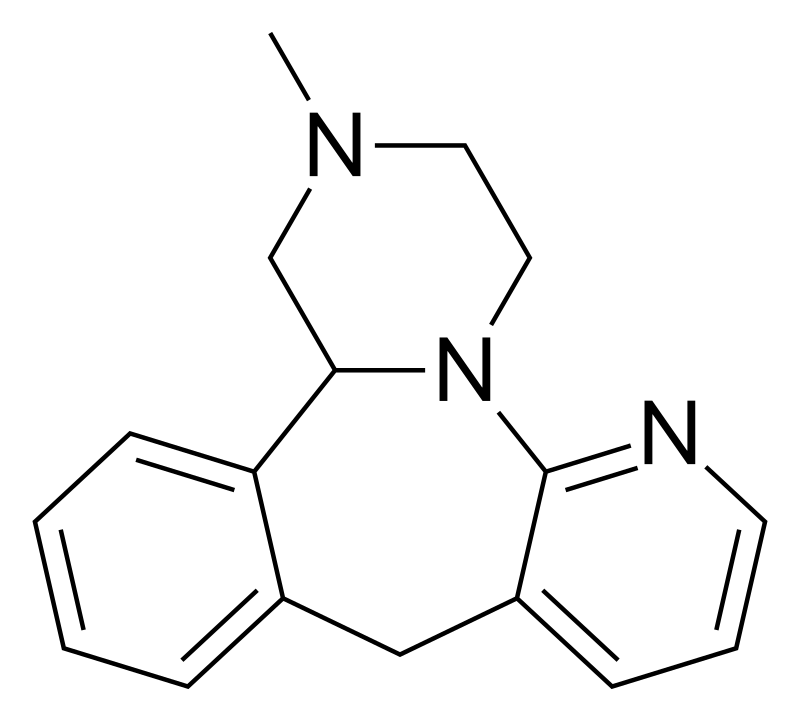
Chemistry
Mirtazapine is a synthetic molecule belonging to the piperazine-azepine and phenethylamine group of compounds. Its structure comprises a fusion of pyridine, benzene, azepine, and piperazine rings, rendering it a tetracyclic antidepressant due to its four-ring configuration. Notably, Mirtazapine is the 6-aza analog of mianserin, which shares similar pharmacological properties.
The mechanism of action of Mirtazapine involves the augmentation of central adrenergic and serotonergic transmission, potentially achieved by functioning as an antagonist at central presynaptic alpha 2 adrenergic inhibitory autoreceptors and heteroreceptors. Additionally, this compound acts as a potent antagonist at 5-hydroxytryptamine type 2 (5-HT2), 5-HT3, and histamine 1 (H1) receptors while displaying moderate antagonist activity at peripheral alpha 1 adrenergic and muscarinic receptors.
Pharmacology
Mirtazapine exerts its pharmacological effects through its interactions with various receptors, acting as an antagonist or inverse agonist on the following receptor types:
- 5-HT2A receptor
- 5-HT2B receptor
- 5-HT2C receptor
- 5-HT3 receptor
- 5-HT7 receptor
- α1-adrenergic receptor
- α2A-adrenergic receptor
- α2B-adrenergic receptor
- α2C-adrenergic receptor
- H1 receptor
- mACH receptors
While Mirtazapine exhibits some affinity for the 5-HT2A receptor, it functions as an antagonist,[6] suggesting that this mechanism is unlikely responsible for its psychedelic and deliriant effects.
Moreover, Mirtazapine has been observed to indirectly activate the following G protein-coupled receptors (GPCRs) in humans:
Opioid receptor κ3
Mirtazapine’s modulation of the κ3 opioid receptor contributes to pain relief[32] and contributes to the soothing and hallucinogenic effects of the compound. This interaction may also explain specific withdrawal or discontinuation effects associated with Mirtazapine, as well as its potential to promote diuresis and increase food intake, often resulting in weight gain.
It’s important to note that while some of these effects are observed in individuals who use Mirtazapine recreationally or in one-off dosing scenarios, most neurophysiological effects manifest in individuals with ongoing use, such as those prescribed 15, 30, or 45 mg daily for conditions like depression. This consistent use leads to a sustained level of Mirtazapine in the body.
Mirtazapine has an oral bioavailability of approximately 50% and is primarily bound to plasma proteins, around 85%. It undergoes metabolism in the liver, primarily via demethylation and hydroxylation mediated by cytochrome P450 enzymes (CYP1A2, CYP2D6, CYP3A4). One of its primary metabolites is desmethyl mirtazapine. The drug has an overall elimination half-life ranging from 20 to 40 hours. In terms of excretion, it is conjugated in the kidney and eliminated in the urine, with approximately 75% of the drug being excreted in this manner. In comparison, around 15% is eliminated in feces.
Subjective effects
Mirtazapine predominantly elicits psychedelic effects, albeit with distinct deliriant-like characteristics. Notably, the hallucinations it induces often possess a convincingly delirious quality, rarely exhibiting condensed visual geometries. Instead, they tend to manifest as solid, highly realistic perceptions.
The cognitive landscape experienced during high-dose mirtazapine use is typically devoid of insight. Unlike many other hallucinogenic substances that may foster introspection, creative thinking, or problem-solving, mirtazapine is generally reported to lack therapeutic potential when employed as a hallucinogen.
Please note that the ensuing effects are derived from the Subjective Effect Index (SEI), which relies on anecdotal user accounts and analyses from contributors on PsychonautWiki. As such, they should be regarded with a degree of skepticism.
Furthermore, these effects may not occur consistently or predictably, but higher doses are more likely to encompass the entire spectrum. Additionally, higher doses come with an increased risk of adverse effects, including addiction, severe injury, or even death ☠.
Physical:
- Sedation: Mirtazapine is profoundly sedating, often inducing an overwhelmingly lethargic state. Users may feel as if they are severely sleep-deprived, on the verge of passing out, and unable to engage in physical activities, with sedation intensifying proportionally with the dosage.
- Spontaneous bodily sensations: The “body high” involves a pleasant, warm, enveloping tingling sensation. It remains consistently present, intensifying as the experience unfolds, though it generally maintains a mildly euphoric character even at high doses. Additionally, mild throbbing or aching sensations may occur.
- Tactile hallucination: Unique tactile hallucinations are common with mirtazapine, characterized by structured vibrations and pulsations spontaneously emerging across the skin and propagating outward from their point of origin.
- Changes in felt gravity
- Motor control loss: Engaging in physical activities such as walking may result in a distinct but not entirely incapacitating loss of motor control, accompanied by a sensation of walking on a trampoline rather than a solid surface.
- Appetite enhancement: Mirtazapine induces a robust increase in appetite, akin in strength to the “munchies” experienced with cannabis.[37]
- Constipation
- Dizziness
- Dry mouth
- Bronchodilation: Swallowing may become challenging and uncomfortable, similar to some other anticholinergics like diphenhydramine. This effect is most prominent during the onset phase and often diminishes as the peak sets in.
- Muscle relaxation
- Restless legs: This effect is slightly less apparent compared to diphenhydramine.
- Nausea suppression
Auditory:
- Auditory distortion
- Auditory hallucination
- Auditory enhancement
Cognitive:
- Thought deceleration
- Analysis suppression
- Cognitive euphoria or Cognitive dysphoria: Recreational use reports describe mild euphoria in some cases, while others report either a neutral state of mind or dysphoria due to pronounced side effects.
- Dream potentiation: Dreams become more vivid than usual.
- Conceptual thinking
- Amnesia
- Immersion enhancement
- Time distortion
- Increased music appreciation
- Irritability
- Emotion suppression: Mirtazapine tends to dampen emotions, making it difficult to express them.
- Anxiety suppression: This effect is more substantial and more rapidly acting than that of SSRIs.
Visual:
- Distortions
- Visual acuity suppression
- Tracers: This effect is quite common and can manifest more frequently at level 4 than with most (if not all) traditional psychedelics.
- Drifting (melting, flowing, breathing, and morphing)
- Color tinting
- Color shifting
- Symmetrical texture repetition
- Visual haze
Geometry:
- In terms of complexity, the visual geometry during high-dose mirtazapine trips can be likened to that of LSD or psilocin. It is intricate, abstract, structured, equally organic and synthetic, dimly lit, monotone in scheme, glossy in shading, with sharp and soft edges, large in size, fast in speed, smooth in motion, and consistent in intensity.
- At higher dosages, Level 8A and Level 8B experiences are rare due to overwhelming sedation. Users may report a menacing and ominous quality, featuring predominantly gray and blue color schemes.
Hallucinatory states:
- External hallucination (Autonomous entities, Settings, sceneries, landscapes, Perspective alterations, Scenarios, and plots): Similar to experiences associated with deliriants but generally occurring only at high dosages. These hallucinations are vivid, autonomous, and believable.
- Internal hallucination (Autonomous entities; settings, sceneries, and landscapes; perspective hallucinations, and scenarios and plots): Generally present as spontaneous breakthroughs at higher dosages, especially when falling asleep. These hallucinations are believable, interactive, novel, and solid in appearance. They often take the form of bizarre and nonsensical plots, frequently occurring during the transition to sleep (hypnagogic states) or upon waking (hypnopompic states).
- Transformations
- Object activation
Combination:
- Cannabis: Combining mirtazapine with cannabis powerfully potentiates euphoric and visual impact.
- Psychedelics: Mirtazapine’s action as a 5-HT2A antagonist may help reduce the intensity of a bad trip or “abort” it.
Toxicity
Mirtazapine is not associated with brain damage and exhibits remarkably low toxicity relative to its dosage. Much like other psychedelic substances, there are few physical side effects linked to mirtazapine when used responsibly. Multiple studies have demonstrated that, when taken in reasonable doses and in a controlled setting, it does not yield adverse cognitive, psychiatric, or toxic physical consequences.
Dependence and abuse potential:
- Mirtazapine does not foster habit formation when utilized as a hallucinogen. In fact, the desire to use it may diminish with continued use, making it an automated substance.
- Tolerance to mirtazapine’s effects develops almost immediately after ingestion. Subsequently, it takes approximately three days for patience to reduce by half and seven days to return to baseline levels, assuming no further consumption.
Overdose:
- Mirtazapine is generally considered safe in the event of an overdose, although its toxicity in overdose is slightly higher than that of most SSRIs (except citalopram). Notably, unlike tricyclic antidepressants (TCAs), mirtazapine does not exhibit significant cardiovascular adverse effects, even at doses 7 to 22 times higher than the maximum recommended amount.
- While case reports have described overdose instances involving up to 30 to 50 times the standard dose, mirtazapine is generally regarded as relatively nontoxic when compared to TCAs.
- There have been twelve reported fatalities attributed to mirtazapine overdose. The fatal toxicity index (deaths per million prescriptions) for mirtazapine is 3.1, which is similar to that observed with SSRIs.
- It is strongly advised to employ harm reduction practices when using this substance.
Dangerous interactions:
- Combining mirtazapine with different types of antidepressants can lead to adverse effects and potentially result in serotonin syndrome.
Legal status
Mirtazapine is approved for medical use worldwide, but its sale and possession without a prescription are generally illegal in most countries. Here are some specific regulations in certain regions:
- Switzerland: In Switzerland, Mirtazapine is categorized as an “Abgabekategorie B” pharmaceutical, typically requiring a prescription.
- Turkey: Mirtazapine is classified as an antidepressant and is available by prescription, although enforcement of this law is sometimes lax.
- United Kingdom: Mirtazapine is a licensed prescription-only medicine (POM) in the United Kingdom. Possessing this medicine without a valid prescription is not a criminal offense. It can be legally obtained with a valid prescription or through legal import for personal use, as outlined in Section 13 of the Medicines Act 1968.
FAQ
- What is Mirtazapine?
- Mirtazapine is an antidepressant medication. It belongs to the class of drugs known as tetracyclic antidepressants and is used to treat depression and various mood disorders. It is available under the brand name Remeron, among others.
- How does Mirtazapine work?
- Mirtazapine works by enhancing the transmission of certain neurotransmitters, such as serotonin and norepinephrine, in the brain. It acts as an antagonist or inverse agonist on various receptors, affecting the balance of these neurotransmitters.
- What are the common medical uses of Mirtazapine?
- Mirtazapine is primarily prescribed to treat major depressive disorder and other mood disorders. It can also be used off-label for conditions like generalized anxiety disorder, social anxiety disorder, obsessive-compulsive disorder, and insomnia, among others.
- Does Mirtazapine have any recreational or hallucinogenic effects?
- While Mirtazapine is not typically used recreationally, some individuals have reported unusual psychedelic and sedative effects at high doses. However, these effects are not the intended use of the medication, and such use is not recommended due to potential risks.
- Is Mirtazapine habit-forming or addictive?
- Mirtazapine is generally not considered habit-forming, and the desire to use it may decrease with use. It is not known for causing addiction when used as prescribed.
- What is the typical dosage of Mirtazapine?
- Dosage can vary based on the individual’s condition and response to the medication. It is important to follow your healthcare provider’s instructions carefully and stay within the prescribed dose.
- How long does it take for Mirtazapine to start working?
- It may take several weeks of regular use before the full therapeutic effects of Mirtazapine are felt. It is essential to continue taking it as directed, even if you have yet to notice immediate improvement.
- What are the potential side effects of Mirtazapine?
- Common side effects of Mirtazapine may include drowsiness, increased appetite, weight gain, and dry mouth. Less common side effects can include dizziness, constipation, and changes in blood pressure. It’s important to discuss any side effects with your healthcare provider.
- Can Mirtazapine be overdosed?
- Mirtazapine is considered relatively safe in the event of an overdose, but like any medication, taking excessive amounts can be harmful. It’s crucial to follow the prescribed dosage and seek immediate medical attention if an overdose is suspected.
- Is Mirtazapine legal?
- Mirtazapine is legally approved for medical use worldwide. However, its sale and possession without a prescription are generally illegal in most countries. Specific regulations may vary by region, so it’s essential to check your local laws and guidelines.
References
- “REMERON (mirtazapine) tablet, film coated [Organon Pharmaceuticals USA]”. DailyMed. Organon Pharmaceuticals USA. October 2012. Retrieved 24 October 2013.
- Schatzberg, A. F., Cole, J. O., DeBattista, C. (2010). Manual of clinical psychopharmacology. 3 (7th ed ed.). American Psychiatric Pub. ISBN 9781585623778.
- Gorman, J. M. (1999). “Mirtazapine: clinical overview”. The Journal of Clinical Psychiatry. 60 Suppl 17: 9–13; discussion 46–48. ISSN 0160-6689.
- “Review of the use of mirtazapine in the treatment of depression”. Expert Opinion on Pharmacotherapy.
- Anttila, S. A. K., Leinonen, E. V. J. (7 June 2006). “A Review of the Pharmacological and Clinical Profile of Mirtazapine”. CNS Drug Reviews. 7 (3): 249–264. doi:10.1111/j.1527-3458.2001.tb00198.x. ISSN 1080-563X.
- Croom, K. F., Perry, C. M., Plosker, G. L. (1 May 2009). “Mirtazapine”. CNS Drugs. 23 (5): 427–452. doi:10.2165/00023210-200923050-00006. ISSN 1179-1934.
- Muehlbacher, M., Nickel, M. K., Nickel, C., Kettler, C., Lahmann, C., Gil, F. P., Leiberich, P. K., Rother, N., Bachler, E., Fartacek, R., Kaplan, P., Tritt, K., Mitterlehner, F., Anvar, J., Rother, W. K., Loew, T. H., Egger, C. (December 2005). “Mirtazapine Treatment of Social Phobia in Women: A Randomized, Double-Blind, Placebo-Controlled Study”. Journal of Clinical Psychopharmacology. 25 (6): 580–583. doi:10.1097/01.jcp.0000186871.04984.8d. ISSN 0271-0749.
- Mirtazapine Treatment of Obsessive-Compulsive Disorder | http://journals.lww.com/psychopharmacology/Citation/2001/10000/Mirtazapine_Treatment_of_Obsessive_Compulsive.16.aspx
- Carpenter, L., Leon, Z., Yasmin, S., Price, L. (1 June 1999). “Clinical Experience with Mirtazapine in the Treatment of Panic Disorder”. Annals of Clinical Psychiatry. 11 (2): 81–86. doi:10.3109/10401239909147053. ISSN 1040-1237.
- Landowski, J. (December 2002). “[Mirtazapine–an antidepressant]”. Psychiatria Polska. 36 (6 Suppl): 125–130. ISSN 0033-2674.
- Chinuck, R. S., Fortnum, H., Baldwin, D. R. (December 2007). “Appetite stimulants in cystic fibrosis: a systematic review”. Journal of Human Nutrition and Dietetics. 20 (6): 526–537. doi:10.1111/j.1365-277X.2007.00824.x. ISSN 0952-3871.
- “Management of symptons associated with advanced cancer: olanzapine and mirtazapine”. Expert Review of Anticancer Therapy.
- Hartmann, P. M. (1 January 1999). “Mirtazapine: a newer antidepressant”. American Family Physician. 59 (1): 159–161. ISSN 0002-838X.
- Jindal, R. D. (1 April 2009). “Insomnia in Patients with Depression”. CNS Drugs. 23 (4): 309–329. doi:10.2165/00023210-200923040-00004. ISSN 1179-1934.
- Nutt, D. J. (June 2002). “Tolerability and safety aspects of mirtazapine”. Human Psychopharmacology: Clinical and Experimental. 17 (S1): S37–S41. doi:10.1002/hup.388. ISSN 0885-6222.
- Li, T.-C., Shiah, I.-S., Sun, C.-J., Tzang, R.-F., Huang, K.-C., Lee, W.-K. (June 2011). “Mirtazapine Relieves Post-Electroconvulsive Therapy Headaches and Nausea: A Case Series and Review of the Literature”. The Journal of ECT. 27 (2): 165–167. doi:10.1097/YCT.0b013e3181e63346. ISSN 1095-0680.
- Kast, R. E., Foley, K. F. (July 2007). “Cancer chemotherapy and cachexia: mirtazapine and olanzapine are 5-HT3 antagonists with good antinausea effects”. European Journal of Cancer Care. 16 (4): 351–354. doi:10.1111/j.1365-2354.2006.00760.x. ISSN 0961-5423.
- Twycross, R. (1 January 2003). “Itch: scratching more than the surface”. QJM. 96 (1): 7–26. doi:10.1093/qjmed/hcg002. ISSN 1460-2393.
- Greaves, M. W. (17 November 2005). “Itch in systemic disease: therapeutic options: Itch in systemic disease”. Dermatologic Therapy. 18 (4): 323–327. doi:10.1111/j.1529-8019.2005.00036.x. ISSN 1396-0296.
- Colombo, B., Annovazzi, P. O. L., Comi, G. (1 October 2004). “Therapy of primary headaches: the role of antidepressants”. Neurological Sciences. 25 (3): s171–s175. doi:10.1007/s10072-004-0280-x. ISSN 1590-3478.
- Tajti, J., Almási, J. (June 2006). “[Effects of mirtazapine in patients with chronic tension-type headache. Literature review]”. Neuropsychopharmacologia Hungarica: A Magyar Pszichofarmakologiai Egyesulet Lapja = Official Journal of the Hungarian Association of Psychopharmacology. 8 (2): 67–72. ISSN 1419-8711.
- NCI Thesaurus – Mirtazapine (Code C29265)
- Fernández, J., Alonso, J. M., Andrés, J. I., Cid, J. M., Díaz, A., Iturrino, L., Gil, P., Megens, A., Sipido, V. K., Trabanco, A. A. (1 March 2005). “Discovery of New Tetracyclic Tetrahydrofuran Derivatives as Potential Broad-Spectrum Psychotropic Agents”. Journal of Medicinal Chemistry. 48 (6): 1709–1712. doi:10.1021/jm049632c. ISSN 0022-2623.
- Boer, Th. de, Maura, G., Raiteri, M., Vos, C. J. de, Wieringa, J., Pinder, R. M. (1 April 1988). “Neurochemical and autonomic pharmacological profiles of the 6-aza-analogue of mianserin, org 3770 and its enantiomers”. Neuropharmacology. 27 (4): 399–408. doi:10.1016/0028-3908(88)90149-9. ISSN 0028-3908.
- Boer, T. de (1996). “The pharmacologic profile of mirtazapine”. The Journal of Clinical Psychiatry. 57 Suppl 4: 19–25. ISSN 0160-6689.
- Goodman, L. S., Brunton, L. L., Chabner, B., Knollmann, B. C., eds. (2011). Goodman & Gilman’s pharmacological basis of therapeutics (12th ed ed.). McGraw-Hill. ISBN 9780071624428.
- TGA eBS – Product and Consumer Medicine Information Licence
- Kennis, L. E., Bischoff, F. P., Mertens, C. J., Love, C. J., Van den Keybus, F. A., Pieters, S., Braeken, M., Megens, A. A., Leysen, J. E. (3 January 2000). “New 2-substituted 1,2,3,4,10,14b-Hexahydro-6-methoxy-2-methyldibenzo[ c , f ]pyrazino[1,2- a ]azepin and Its Enantiomers in Comparison with the Two Antidepressants Mianserin and Mirtazapine”. Journal of Medicinal Chemistry. 45 (15): 3280–3285. doi:10.1021/jm010566d. ISSN 0022-2623.
- Schreiber, S., Rigai, T., Katz, Y., Pick, C. G. (September 2002). “The antinociceptive effect of mirtazapine in mice is mediated through serotonergic, noradrenergic and opioid mechanisms”. Brain Research Bulletin. 58 (6): 601–605. doi:10.1016/S0361-9230(02)00825-0. ISSN 0361-9230.
- Dapoigny, M., Abitbol, J. L., Fraitag, B. (October 1995). “Efficacy of peripheral kappa agonist fedotozine versus placebo in treatment of irritable bowel syndrome. A multicenter dose-response study”. Digestive Diseases and Sciences. 40 (10): 2244–2249. doi:10.1007/BF02209014. ISSN 0163-2116.
- Pande, A. C., Pyke, R. E., Greiner, M., Wideman, G. L., Benjamin, R., Pierce, M. W. (October 1996). “Analgesic efficacy of enadoline versus placebo or morphine in postsurgical pain”. Clinical Neuropharmacology. 19 (5): 451–456. doi:10.1097/00002826-199619050-00009. ISSN 0362-5664.
- Rimoy, G. H., Wright, D. M., Bhaskar, N. K., Rubin, P. C. (1994). “The cardiovascular and central nervous system effects in the human of U-62066E. A selective opioid receptor agonist”. European Journal of Clinical Pharmacology. 46 (3): 203–207. doi:10.1007/BF00192549. ISSN 0031-6970.
- Al-Majed, A., Bakheit, A. H., Alharbi, R. M., Abdel Aziz, H. A. (2018). “Mirtazapine”. Profiles of Drug Substances, Excipients, and Related Methodology. 43: 209–254. doi:10.1016/bs.podrm.2018.01.002. ISSN 1871-5125.
- Schatzberg, A. F., Nemeroff, C. B., eds. (2009). The American Psychiatric Publishing textbook of psychopharmacology (4th ed ed.). American Psychiatric Pub. ISBN 9781585623099.
- Website Link: Mirtazapine Side Effects
- Taylor, D., Paton, C., Kapur, S., eds. (2012). The Maudsley prescribing guidelines in psychiatry (11. ed ed.). Wiley. ISBN 9780470979488.
- White N, Litovitz T, Clancy C (December 2008). “Suicidal antidepressant overdoses: a comparative analysis by antidepressant type” (PDF). Journal of Medical Toxicology. 4 (4): 238–50. doi:10.1007/bf03161207. PMC 3550116 Freely accessible. PMID 19031375.
- Fawcett, J., Barkin, R. L. (1 December 1998). “Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression”. Journal of Affective Disorders. 51 (3): 267–285. doi:10.1016/S0165-0327(98)00224-9. ISSN 0165-0327.
- Holzbach R, Jahn H, Pajonk FG, Mähne C (November 1998). “Suicide attempts with mirtazapine overdose without complications”. Biological Psychiatry. 44 (9): 925–6. doi:10.1016/S0006-3223(98)00081-X. PMID 9807651.Template:Unreliable medical source
- Retz W, Maier S, Maris F, Rösler M (November 1998). “Non-fatal mirtazapine overdose”. International Clinical Psychopharmacology. 13 (6): 277–9. doi:10.1097/00004850-199811000-00007. PMID 9861579.Template:Unreliable medical source
- Nikolaou P, Dona A, Papoutsis I, Spiliopoulou C, Maravelias C. “Death Due to Mirtazapine Overdose”. in “Abstracts of the XXIX International Congress of the European Association of Poison Centres and Clinical Toxicologists, May 12–15, 2009, Stockholm, Sweden”. Clinical Toxicology. 47 (5): 436–510. 2009. doi:10.1080/15563650902952273.
- Baselt, RC (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 1045–7. ISBN 978-0-9626523-7-0.
- Buckley NA, McManus PR (December 2002). “Fatal toxicity of serotoninergic and other antidepressant drugs: analysis of United Kingdom mortality data”. BMJ. 325 (7376): 1332–3. doi:10.1136/bmj.325.7376.1332. PMC 137809 Freely accessible. PMID 12468481.Template:Unreliable medical source
- MHRA (November 10, 2006). “MHRA license for Modafinil in UK” (PDF). MHRA.
- “Medicines Act 1968 Section 13”.