Contents
Summary
DET, scientifically referred to as N, N-diethyltryptamine, and occasionally known as T-9, is a psychedelic substance with close ties to DMT and 4-HO-DET. Notably, despite its structural resemblance to DMT, DET’s psychoactive effects can be triggered by an oral dose of approximately 50-100 mg, and it operates without the need for MAO inhibitors. The effects typically endure for a duration of approximately 2 to 4 hours.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 61-51-8 |
|---|---|
| PubChem CID | 6090 |
| DrugBank | DB01460 |
| ChemSpider | 5865 |
| UNII | 916E8V4S2V |
| ChEMBL | ChEMBL142936 |
| CompTox Dashboard (EPA) | DTXSID9052763 |
| Chemical and physical data | |
| Formula | C14H20N2 |
| Molar mass | 216.328 g·mol−1 |
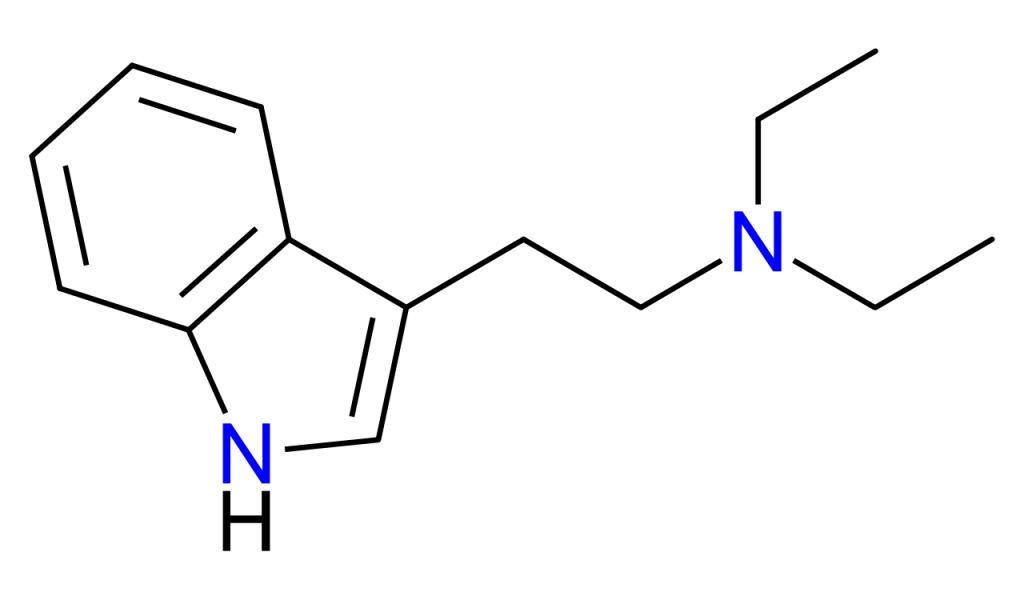
Chemistry
DET is an analogue of the common tryptamine hallucinogen N,N-Dimethyltryptamine or DMT.
Pharmacology
The mechanism of action is believed to involve serotonin receptor agonism, akin to the mode of action seen in other classical psychedelics.
DET is occasionally favoured over DMT due to its suitability for oral administration, a route not available with DMT. The reason for this distinction lies in the fact that the enzyme monoamine oxidase degrades DMT into an inactive compound before it can be effectively absorbed. To overcome this enzymatic hurdle, DMT must be administered through alternative means, such as intravenous injection, intramuscular injection, inhalation, insufflation, rectal administration, or oral ingestion in conjunction with a monoamine oxidase inhibitor. In contrast, DET’s structure, with ethyl groups attached to its nitrogen atom, renders it impervious to degradation by monoamine oxidase. This characteristic extends to several other tryptamines featuring larger nitrogen substituents.
Biochemistry
While DET is a synthetic compound without established natural origins, it has been employed in combination with Psilocybe cubensis mycelium to synthesize artificial substances, specifically 4-PO-DET (Ethocybin) and 4-HO-DET (Ethocin). This contrasts with the naturally transpiring 4-PO-DMT (Psilocybin) and 4-HO-DMT (Psilocin). Through the isolation process of alkaloids, the yield included 3.3% for 4-HO-DET and a range of 0.01-0.8% for 4-PO-DET.
Psychosis model
In the initial investigations into DET and other psychedelics, considerable attention was placed on their suspected psychotomimetic characteristics. Researchers posited the idea that aberrant metabolites of naturally occurring compounds like tryptamine, serotonin, and tryptophan might hold the key to understanding conditions like schizophrenia or psychosis. However, as scientific knowledge and pharmacological comprehension have advanced, this theory has largely been refuted by the majority of researchers.
Legal status
Internationally DET is a Schedule I drug under the Convention on Psychotropic Substances.
FAQ
1. What is Diethyltryptamine (DET)?
Diethyltryptamine (DET) is a synthetic psychedelic compound that belongs to the tryptamine class of chemicals. It is structurally related to other well-known psychedelics like DMT.
2. How does DET compare to DMT in terms of administration?
One notable difference between DET and DMT is that DET can be taken orally, while DMT is typically not orally active. The enzymatic action of monoamine oxidase (MAO) degrades DMT when ingested, making alternative methods of administration necessary for DMT.
3. What is the mechanism of action for DET?
DET is believed to work through serotonin receptor agonism, similar to the mechanism of action seen in other classic psychedelics.
4. Is DET found in nature, or is it entirely synthetic?
DET is a synthetic compound with no known natural sources. It is produced through chemical synthesis.
5. How has DET been used in conjunction with Psilocybe cubensis mycelium?
DET has been employed along with Psilocybe cubensis mycelium to synthesize synthetic substances, such as 4-PO-DET (Ethocybin) and 4-HO-DET (Ethocin). These synthetic compounds differ from naturally occurring psilocybin and psilocin, which are found in psychedelic mushrooms.
6. What is the history of research on DET?
Early studies on DET and similar psychedelics focused on their potential psychotomimetic properties. Researchers explored the idea that unusual metabolites of endogenous compounds like tryptamine, serotonin, and tryptophan might be connected to conditions such as schizophrenia or psychosis. However, this theory has been largely dismissed by most researchers as our understanding of pharmacology and mental disorders has advanced.
7. Is DET currently used for therapeutic purposes?
DET’s therapeutic potential has not been extensively studied, and it is not currently employed as a therapeutic substance. The use of any psychedelic for therapeutic purposes should only occur under the guidance of qualified medical professionals and in clinical settings.
8. What is the legal status of DET?
The legal status of DET varies by country and region. It’s important to be aware of and comply with local laws and regulations regarding its use and distribution.
9. Where can I find more information about DET?
For additional information on DET, you can refer to scientific literature academic research, or consult experts in the field of psychoactive substances. Always prioritize safety and responsible use when considering the consumption of any such compounds.
References
- Anvisa, in its resolution RDC Nº 804, published in Brazilian Portuguese on July 25, 2023, delineated the Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control. This resolution was archived from the original source on August 27, 2023, and can be accessed through the official Diário Oficial da União.
- The Erowid DET Vault provides valuable insights into the chemistry of DET, offering a resource for those seeking information about this compound. This online resource was last retrieved on January 8, 2008.
- In September 1969, a study conducted by Winter JC explored the behavioral effects of N,N-diethyltryptamine (DET). The research, titled “Behavioral effects of N,N-diethyltryptamine: absence of antagonism by xylamidine tosylate,” was featured in The Journal of Pharmacology and Experimental Therapeutics (Volume 169, Issue 1), with reference to PMID 5306645.
- In 1989, a study by Gartz delved into the biotransformation of tryptamine derivatives within mycelial cultures of Psilocybe. The research findings were documented in the Journal of Basic Microbiology (Volume 29, Issue 6) and can be accessed through DOI doi:10.1002/jobm.3620290608, with reference to PMID 2614674.
- In 1960, Böszörmenyi contributed to the understanding of psilocybin and diethyltryptamine (DET) as two tryptamine hallucinogens in the field of neuro-psychopharmacology. The research was presented in the publication “Neuro-psychopharm” (Volume 2, pages 226–229).
- A study from April 1965 by Khazan and McCash explored the effects of LSD-25, n,n-dimethyltryptamine (DMT), and N,N-diethyltryptamine (DET) on the photic evoked responses in unanesthetized rabbits. This research was featured in “Archives Internationales de Pharmacodynamie et de Therapie” (Volume 154, Issue 2) and is referenced under PMID 5839429.
- The International Narcotics Control Board, in August 2003, released a “List of psychotropic substances under international control.” This list provides valuable information on controlled psychotropic substances and their regulatory status. The original document is archived and can be accessed online.
- The “Poisons Standard,” as documented by the Australian Government in October 2015, provides regulatory information on various substances. This standard outlines the classification and control measures associated with different substances, including DET.