Contents
Summary
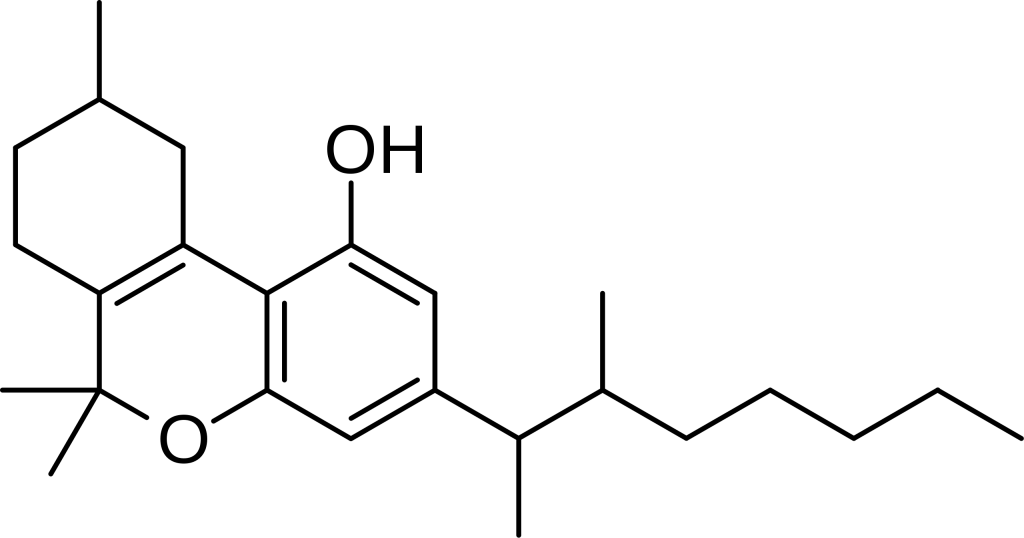
Dimethylheptylpyran, also known as DMHP, 3-(1,2-dimethylheptyl)-Δ6a(10a)-THC, 1,2-dimethylheptyl-Δ3-THC, A-40824, or EA-2233, represents a synthetic analogue of THC. This compound was first developed in 1949 as part of scientific efforts aimed at unraveling the structure of Δ9-THC, one of the primary active constituents found in Cannabis.
DMHP presents itself as a pale yellow, viscous oil that exhibits insolubility in water while readily dissolving in alcohol or non-polar solvents.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 32904-22-6 |
|---|---|
| PubChem CID | 36276 |
| ChemSpider | 33359 |
| UNII | 944O1KA97G |
| ChEMBL | ChEMBL3244434 |
| CompTox Dashboard (EPA) | DTXSID60954555 |
| Chemical and physical data | |
| Formula | C25H38O2 |
| Molar mass | 370.577 g·mol−1 |
Effects
DMHP shares a structural resemblance with THC, primarily differing in the arrangement of a single double bond and the substitution of the 3-pentyl chain with a 3-(1,2-dimethylheptyl) chain. This results in comparable effects to THC, including sedative properties. However, DMHP is notably more potent, particularly in terms of its analgesic and anticonvulsant effects, while exhibiting relatively milder psychological effects. It is believed to function as a CB1 agonist, akin to other cannabinoid derivatives. While DMHP itself has received limited research attention since the characterization of cannabinoid receptors, its structural isomer, 1,2-dimethylheptyl-Δ8-THC, has demonstrated significant potency as a cannabinoid agonist, with separate investigations into the activity of its enantiomers.
Investigation as non-lethal incapacitating agent
The US military’s chemical weapons program at Edgewood Arsenal conducted extensive research on DMHP and its O-acetate ester as potential non-lethal incapacitating agents.
DMHP possesses three stereocenters, resulting in eight distinct stereoisomers, each varying significantly in potency. The collective mixture of these eight O-acetyl ester isomers was assigned the code name EA-2233, with individual isomers designated EA-2233-1 through EA-2233-8. The most potent among them is EA-2233-2, with an effective dose range in humans of 0.5–2.8 μg/kg (approximately 35–200 μg for a 70 kg adult). While individual responses to active doses varied, doses of 1–2 mg of EA-2233 rendered all volunteers unfit for military duties, and the effects persisted for up to 2–3 days.
DMHP undergoes a metabolic process similar to THC, generating the active metabolite 11-hydroxy-DMHP. Notably, DMHP exhibits even greater lipophilicity than THC, resulting in an extended duration of action and a prolonged half-life in the body, ranging from 20 to 39 hours, with the metabolite’s half-life exceeding 48 hours.
While DMHP and its esters induce sedation and mild hallucinogenic effects akin to high doses of THC, they also trigger pronounced hypotension (low blood pressure) at doses below the hallucinogenic threshold. This can lead to severe symptoms such as dizziness, fainting, ataxia, and muscle weakness, making it challenging to stand upright or engage in vigorous physical activities.
The acute toxicity of DMHP was found to be low in both human and animal studies, with a therapeutic index (the ratio of ED50 to LD50) in animals measuring approximately 2000 times. There have been no reported deaths attributed to any of the DMHP EA-2233 stereoisomers 1–8, only symptoms consistent with the most severe levels of THC intoxication. DMHP has an intravenous LD50 of 63 mg/kg in mice and an intravenous minimal lethal dose of 10 mg/kg in dogs.
Unsuitability for military application
The Edgewood Arsenal research team concluded that DMHP and its derivatives, particularly the O-acetyl ester of the most potent isomer, EA-2233-2, offered a promising blend of incapacitating effects and a favorable safety profile within their research program.
Nonetheless, DMHP had a drawback, occasionally inducing severe hypotension at doses below those causing incapacitation, a side effect not observed with more widely studied and well-publicized belladonna anticholinergic agents like 3-Quinuclidinyl benzilate (BZ), which had been discovered and subsequently weaponized. The military’s exploration of synthetic cannabis was constrained due to its illegality and political sensitivity, making it challenging to investigate among enlisted servicemen. Both EA-2233-2 and its precursor, the red-oil THC distillate (referred to as EA-1476), received limited financial resources compared to the study of other incapacitating agents, including BZ derivatives and EA 1729 (LSD), which was widely perceived at the time as a potential tool for mind control and truth serum applications during the Cold War.
Initially, the eight stereoisomers of EA-2233 could not be separated. Later attempts focused on isolating and testing two individual isomers of EA-2233. Still, they were found to induce orthostatic hypotension and had minimal effects on performance at the very low doses used. EA-2233 did not appear to possess the required potency to be of military interest, as an oral dose of 60mcg/kg resulted in a maximum decline of only 40% (at most) in performance during language and number processing tasks. A study was later published (citation needed) indicating that the oral effects of ordinary THC were only about one-third as potent as smoked THC from marijuana. This study suggested that the effectiveness of EA-2233 via aerosol delivery might be significantly more significant than the oral route, but this has not been independently confirmed using DMHP EA-2233.
EA-2233 has exclusively been employed within the confines of Edgewood Arsenal, where it caused no fatalities among healthy military volunteers. However, there were concerns that it might pose a risk to very young or elderly individuals or potentially impair motor skills in those with pre-existing hypotension. In all cases, it was reported that subjects showed no lingering effects, flashbacks, or other issues after 72 hours.
Nonetheless, due to concerns about its relative lack of safety, particularly in comparison to BZ, DMHP’s research was halted in favor of exploring BZ for military applications.
Edgewood Arsenal and EA 2233
The fiscal allocation and strategic planning for Edgewood Arsenal, established in 1948, primarily designated it as a defensive research facility. During that era, the U.S. military recognized that the USSR was investing significantly more in chemical weapons development, approximately ten times the U.S. budget. Edgewood initially operated with a somewhat unspecified mandate and limited oversight. The Edgewood Arsenal Chemical Corps was entrusted with the responsibility of ensuring that the United States was adequately prepared to counter potential threats and could respond with its psychochemical deterrent if necessary. Edgewood conducted analyses and provided data to military commanders, enabling them to consider the integration of these findings into their strategic planning. In practice, this effort primarily served to inform strategists about potential weapons and tactics that the enemy might employ.
The Edgewood Laboratory was established in 1948. The original cannabinoid distillate, the precursor to EA 2233, known as EA-1476 or “Red oil,” was first synthesized in 1949. The laboratory research on EA-1476 took place in the mid-1950s. A single batch of EA 2233 was synthesized in 1962 by chemist Harry Pars of A.D. Little Labs under a top-secret government contract. It was administered to groups of enlisted servicemen who had provided informed consent, led by Dr. James Ketchum in 1962. In 1975, the Edgewood laboratory was closed down. Government funding for the ongoing military development of synthetic cannabis became scarce, leading to the suspension of the cannabinoid research program, along with the broader Edgewood Arsenal experiments in the late 1970s. This decision was influenced by various factors, including growing public mistrust of the military and government, as well as the diminishing practical utility of further chemical incapacitating agent development during and after the Vietnam era.
The experiments involving EA 2233 faced criticism from multiple newspapers. Public perception of cannabis and cannabinoids as hazardous and addictive substances has persisted since the 1930s. DMHP was compared to B.Z., a non-cannabis chemical considered impractical by military planners, and was only tested once in a hastily organized operation known as “Project Dork,” a part of Project 112.
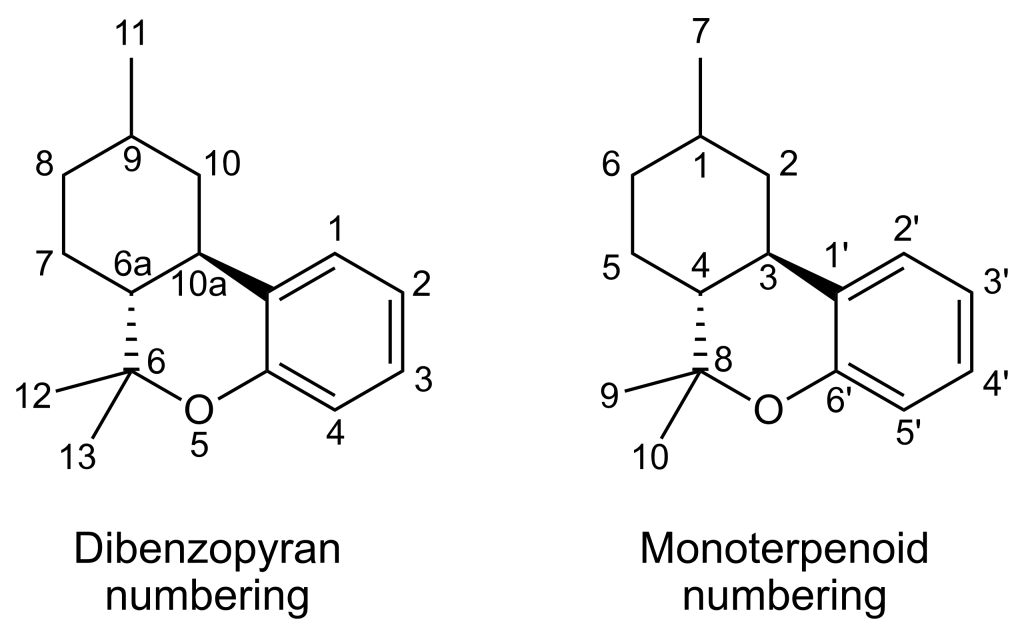
| 7 double bond isomers of dimethylheptylpyran and their 120 stereoisomers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dibenzopyran numbering | Monoterpenoid numbering | Additional chiral centers on side chain | Number of stereoisomers | Natural occurrence | Convention on Psychotropic Substances Schedule | ||||
| Short name | Chiral centers in dibenzopyran backbone | Full name | Short name | Chiral centers in dibenzopyran backbone | 1,2-dimethylheptyl numbering | 3-methyloctan-2-yl numbering | |||
| Δ6a(7)-DMHP | 9 and 10a | 3-(1,2-dimethylheptyl)-8,9,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol | Δ4-DMHP | 1 and 3 | 1 and 2 | 2 and 3 | 16 | No | unscheduled |
| Δ7-DMHP | 6a, 9 and 10a | 3-(1,2-dimethylheptyl)-6a,9,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol | Δ5-DMHP | 1, 3 and 4 | 1 and 2 | 2 and 3 | 32 | No | unscheduled |
| Δ8-DMHP | 6a and 10a | 3-(1,2-dimethylheptyl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol | Δ6-DMHP | 3 and 4 | 1 and 2 | 2 and 3 | 16 | No | unscheduled |
| Δ9,11-DMHP | 6a and 10a | 3-(1,2-dimethylheptyl)-6a,7,8,9,10,10a-hexahydro-6,6-dimethyl-9-methylene-6H-dibenzo[b,d]pyran-1-ol | Δ1(7)-DMHP | 3 and 4 | 1 and 2 | 2 and 3 | 16 | No | unscheduled |
| Δ9-DMHP | 6a and 10a | 3-(1,2-dimethylheptyl)-6a,7,8,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol | Δ1-DMHP | 3 and 4 | 1 and 2 | 2 and 3 | 16 | No | unscheduled |
| Δ10-DMHP | 6a and 9 | 3-(1,2-dimethylheptyl)-6a,7,8,9-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol | Δ2-DMHP | 1 and 4 | 1 and 2 | 2 and 3 | 16 | No | unscheduled |
| Δ6a(10a)-DMHP | 9 | 3-(1,2-dimethylheptyl)-7,8,9,10-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol | Δ3-DMHP | 1 | 1 and 2 | 2 and 3 | 8 | No | Schedule I |
FAQ
- What is Dimethylheptylpyran (DMHP)?
- Dimethylheptylpyran (DMHP) is a synthetic cannabinoid compound. It is structurally similar to delta-9-tetrahydrocannabinol (THC), the primary psychoactive component in cannabis, but with distinct differences in chemical structure.
- How does DMHP compare to THC in terms of effects?
- DMHP produces effects similar to THC, such as sedation and mild hallucinogenic effects. However, DMHP is considerably more potent than THC, especially in terms of its analgesic and anticonvulsant properties, while it tends to induce comparatively weaker psychological effects.
- How is DMHP believed to work in the body?
- DMHP is thought to act as a cannabinoid receptor type 1 (CB1) agonist, much like other cannabinoid derivatives. This interaction with CB1 receptors in the brain and throughout the body is believed to underlie its effects.
- Has DMHP been extensively studied?
- DMHP has received relatively little research attention since the characterization of cannabinoid receptors. However, one of its structural isomers, 1,2-dimethylheptyl-Δ8-THC, has been shown to be a highly potent cannabinoid agonist, and the activities of its enantiomers have been studied separately.
- What were its potential military applications?
- The US military investigated DMHP and its O-acetate ester as possible non-lethal incapacitating agents. EA-2233, the O-acetyl ester of DMHP’s isomers, was identified as one of the more promising agents within the research program.
- Why was DMHP not widely adopted for military use?
- DMHP had the drawback of occasionally causing severe hypotension (low blood pressure) at pre-incapacitating doses, which was not observed with other chemical agents like BZ. Additionally, political and legal constraints limited the military’s exploration of synthetic cannabinoids.
- Are there safety concerns with DMHP?
- The acute toxicity of DMHP was found to be low in both human and animal studies, with a relatively high therapeutic index. No deaths have been attributed to DMHP use, but it can cause pronounced hypotension and other adverse effects.
- Is DMHP still used or studied today?
- DMHP’s research was suspended mainly in the late 1970s, and it is not commonly studied or used today, primarily due to its safety concerns and the availability of other chemical agents for military applications.
- What are the long-term effects of DMHP?
- Based on historical research, it was reported that subjects experienced no detectable residual effects, flashbacks, or long-term consequences after the use of DMHP within 72 hours. However, there needs to be more contemporary data available regarding long-term effects.
- Is DMHP available for recreational use or medical purposes?
- DMHP is not available for recreational or medical use, and it is not a recognized medication or substance for therapeutic applications. It is considered a controlled substance and is illegal in many jurisdictions.
References
- Anvisa’s Regulatory Update (2023)Anvisa (2023-07-24). “RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” [Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- Early Research on Cannabinoid Analogues (1949)Adams R, Harfenist M, Loewe S (1949). “New Analogs of Tetrahydrocannabinol. XIX”. Journal of the American Chemical Society. 71 (5): 1624–1628. doi:10.1021/ja01173a023.
- Total Synthesis of Cannabinoids (1980)Razdan RK (1980). “The Total Synthesis of Cannabinoids”. Total Synthesis of Natural Products. Total Synthesis of Natural Products. Vol. 4. Wiley-Interscience. pp. 185–262. doi:10.1002/9780470129678.ch2. ISBN 978-0-471-05460-3.
- Effects of Cannabinoids on Cortical Activity (1982)Wilkison DM, Pontzer N, Hosko MJ (July 1982). “Slowing of cortical somatosensory evoked activity by delta 9-tetrahydrocannabinol and dimethylheptylpyran in alpha-chloralose-anesthetized cats”. Neuropharmacology. 21 (7): 705–9. doi:10.1016/0028-3908(82)90014-4. PMID 6289158. S2CID 35663464.
- Cannabinoid Derivatives Research (1976)Winn M, Arendsen D, Dodge P, Dren A, Dunnigan D, Hallas R, Hwang K, Kyncl J, Lee YH, Plotnikoff N, Young P, Zaugg H (April 1976). “Drugs derived from cannabinoids. 5. delta6a,10a-Tetrahydrocannabinol and heterocyclic analogs containing aromatic side chains”. Journal of Medicinal Chemistry. 19 (4): 461–71. doi:10.1021/jm00226a003. PMID 817021.
- Cannabinoid Agonists and Conditioned Response (2003)Parker LA, Mechoulam R (2003). “Cannabinoid agonists and antagonists modulate lithium-induced conditioned gaping in rats”. Integrative Physiological and Behavioral Science. 38 (2): 133–45. doi:10.1007/BF02688831. PMID 14527182. S2CID 38974868.
- Synthesis of Potent Cannabinoids (1997)Huffman JW, Duncan Jr SG, Wiley JL, Martin BR (1997). “Synthesis and pharmacology of the 1′,2′-dimethylheptyl-Δ8-THC isomers: exceptionally potent cannabinoids”. Bioorganic & Medicinal Chemistry Letters. 7 (21): 2799–2804. doi:10.1016/S0960-894X(97)10086-5.
- Long-Term Health Effects of Chemical Agents (1984)”Possible Long-Term Health Effects of Short-Term Exposure To Chemical Agents”. Cholinesterase Reactivators, Psychochemicals and Irritants and Vesicants. Vol. 2. Commission on Life Sciences. The National Academies Press. 1984. pp. 79–99. doi:10.17226/9136. ISBN 978-0-309-07772-9.
- Insights into Chemical Warfare (2006)Ketchum JS (2006). “Chapter 5”. Chemical Warfare: Secrets Almost Forgotten. Santa Rosa, CA: ChemBooks Inc. p. 38. ISBN 978-1-4243-0080-8.
- Historical Perspectives on Chemical WarfareKhatchadourian R (12 December 2012). “War of the Mind”. The New Yorker. Retrieved 2021-05-08.