Summary
HU-210, a synthetic cannabinoid, was initially synthesized in 1988 by a research team led by Raphael Mechoulam at the Hebrew University. It boasts an astonishing potency, ranging from 100 to 800 times greater than naturally occurring THC found in cannabis. Additionally, HU-210 exhibits a significantly prolonged duration of action.
In terms of its receptor interactions, HU-210 demonstrates a remarkable binding affinity, with a value of 0.061nM at CB1 and 0.52nM at CB2 in cloned human cannabinoid receptors. To put this into perspective, it far surpasses Delta-9-THC, which exhibits a binding affinity of 40.7nM at CB1.
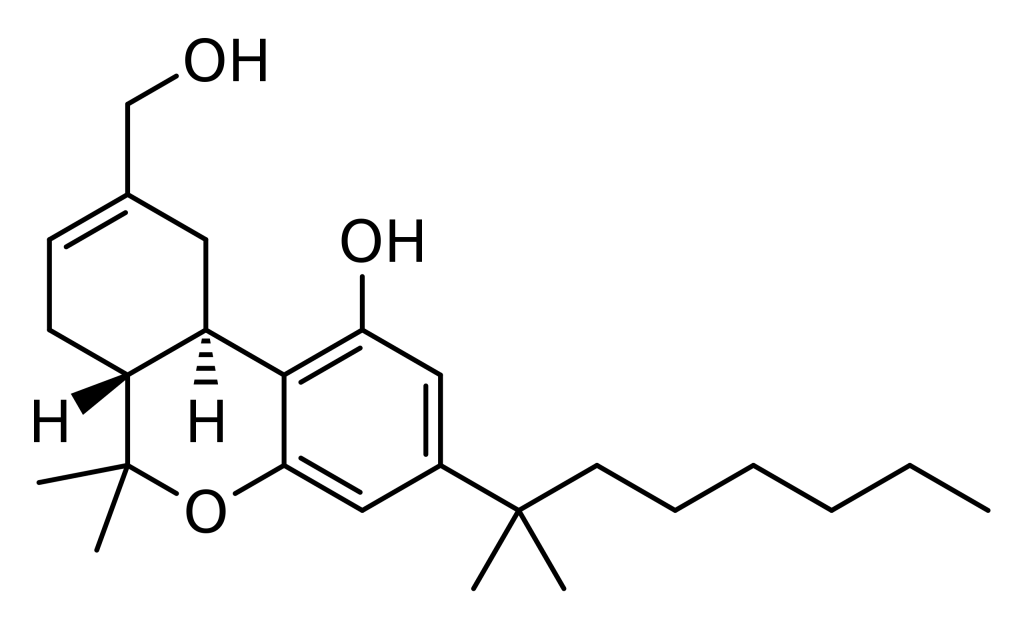
Furthermore, HU-210 can be described as the (–)-1,1-dimethylheptyl analogue of 11-hydroxy-Δ8-tetrahydrocannabinol. In some references, it is alternatively referred to as 1,1-dimethylheptyl-11-hydroxytetrahydrocannabinol. The abbreviation “HU” in its name signifies its origin at the Hebrew University.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 112830-95-2 |
|---|---|
| PubChem CID | 9821569 |
| IUPHAR/BPS | 731 |
| ChemSpider | 7997318 |
| UNII | 191042422P |
| ChEMBL | ChEMBL307696 |
| CompTox Dashboard (EPA) | DTXSID30150188 |
| Chemical and physical data | |
| Formula | C25H38O3 |
| Molar mass | 386.576 g·mol−1 |
Effects and research
HU-210, the (-) enantiomer of 11-OH-D8-THC-DMH, possesses nearly all of the cannabinoid activity, while its counterpart, the (+) enantiomer called HU-211, lacks cannabinoid properties and instead functions as an NMDA antagonist with neuroprotective effects.
Regarding toxicity, HU-210 exhibits an oral LD50 of 5,000mg/kg in rats and 14,200mg/kg in rabbits. The LDLO (Lowest Lethal Dose amount) for HU-210 in humans is 143mg/kg. By comparison, caffeine’s LD50 is estimated to be in the range of 150 to 200 milligrams per kilogram. The LD50 for Delta-8-THC has not been definitively established; a study from 1973 found that doses as high as 9,000mg/kg of Delta-8-THC were nonlethal in monkeys and dogs.
Chemistry
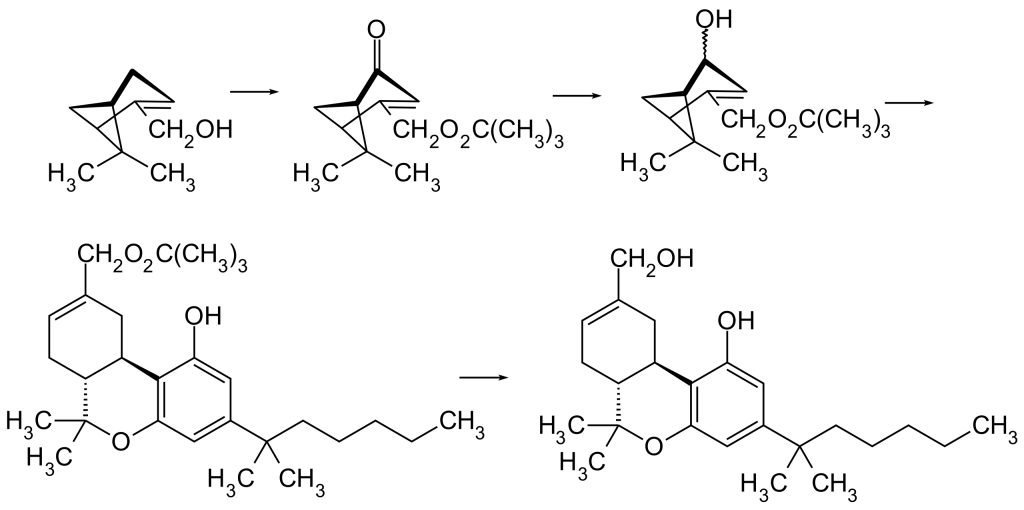
HU-210 is the mirror-image counterpart of HU-211, also known as Dexanabinol. The initial synthesis of HU-210 relies on an acid-catalyzed condensation process involving (–)-Myrtenol and 1,1-Dimethylheptylresorcinol (3,5-Dihydroxy-1-(1,1-dimethylheptyl)benzol).
Legal status
International Regulations:
- HU-210 is not included in the schedules established by the United Nations Single Convention on Narcotic Drugs of 1961 or their Convention on Psychotropic Substances of 1971. As a result, signatory countries to these international drug control treaties are not obligated to regulate HU-210.[14]
National Regulations:
2. New Zealand: As of May 8, 2014, HU-210 is prohibited in New Zealand.
- United States: HU-210 is not explicitly listed among the scheduled controlled substances in the USA. However, a 2009 profile of HU-210 published by the Drug Enforcement Administration (DEA) classified it as a Schedule I controlled substance under the Controlled Substances Act due to its similarity to THC. A 2013 version of this document, now available in PDF format on the DEA Office of Diversion Control’s website, reaffirms HU-210’s Schedule I status, grouping it under ‘tetrahydrocannabinol.’ HU-210 is assigned DEA No. 7370, the same number used for dronabinol and synthetic Delta-8-THC.
- Alabama: HU-210 is categorized as a Schedule I controlled substance in Alabama.
- Florida: HU-210 is listed as a Schedule I controlled substance in Florida, classified as a hallucinogen, making its purchase, sale, or possession illegal in the state without a license.
- Vermont: Effective January 1, 2016, HU-210 is classified as a regulated drug in Vermont and falls under the category of “Hallucinogenic Drug.”
FAQ
- What is HU-210? HU-210 is a synthetic cannabinoid that was developed in 1988. It is known for its high potency and its structural similarity to THC (tetrahydrocannabinol), the active compound in cannabis.
- How does HU-210 differ from THC? HU-210 is significantly more potent than natural THC found in cannabis, with estimates suggesting it can be 100 to 800 times stronger. It also has a longer duration of action.
- What are the effects of HU-210? Like THC, HU-210 produces effects similar to those of natural cannabis, including psychoactive and sedative effects. However, it is known for its considerably stronger analgesic (pain-relieving) and anticonvulsant effects.
- Is HU-210 legal? The legal status of HU-210 varies by country and region. In some places, it is classified as a controlled substance due to its similarity to THC and its psychoactive effects. Always check your local laws and regulations.
- Is HU-210 used for medical purposes? While HU-210 has shown promise in medical research due to its potential analgesic and anticonvulsant effects, its legal status and potency have limited its medical applications.
- What is the LD50 of HU-210? The LD50 (lethal dose for 50% of the population) of HU-210 varies depending on the species and administration method. For example, in rats, it has an oral LD50 of 5,000mg/kg.
- Is HU-210 related to HU-211? HU-210 is the (-) enantiomer of 11-OH-D8-THC-DMH, while HU-211, also known as Dexanabinol, is the (+) enantiomer. HU-211 is not a cannabinoid and has different effects as an NMDA antagonist.
- Can HU-210 be used recreationally? Due to its high potency and legal restrictions in many places, HU-210 is not commonly used recreationally. It is considered a controlled substance in several regions.
- What are the risks associated with HU-210 use? The use of HU-210 can pose risks similar to those associated with THC, including psychoactive effects. Additionally, its high potency can lead to more pronounced and potentially adverse reactions.
- Is HU-210 related to natural cannabis? HU-210 is a synthetic compound inspired by the structure of natural THC found in cannabis. While it shares similarities, it is a distinct synthetic cannabinoid.
References
- Synthesis of Distinct Enantiomers: In 1990, researchers, including Raphael Mechoulam, synthesized distinct enantiomers of a tetrahydrocannabinol derivative.
- Stereospecificity of Psychotropic Activity: In 1988, a study led by Mechoulam investigated the stereospecificity of psychotropic activity of enantiomeric cannabinoids.
- Effects on Mice and Dogs: The stereochemical effects of 11-OH-delta 8-THC-dimethylheptyl on mice and dogs were examined in a study from 1989.
- Discriminative Stimulus Functions: The study of the discriminative stimulus functions of the dimethylheptyl homologs of 11-hydroxy-delta 8-tetrahydrocannabinol in rats and pigeons was conducted in 1989.
- Novel Probe for the Cannabinoid Receptor: In 1992, researchers, including Raphael Mechoulam, introduced a novel probe for the cannabinoid receptor.
- Medicinal Chemistry Endeavors: A 2007 study delved into medicinal chemistry efforts related to phytocannabinoids.
- Structure-Function Relationships: Researchers explored the structure-function relationships of classical cannabinoids in 2016, including CB1/CB2 modulation.
- Inhibition of Adenylate Cyclase: The stereochemical effects of 11-OH-delta 8-tetrahydrocannabinol-dimethylheptyl in inhibiting adenylate cyclase and binding to the cannabinoid receptor were studied in 1990.
- Neuroprotective Properties: In 2003, Dexanabinol, a novel cannabinoid with neuroprotective properties, was discussed.
- Safety Data Sheet: A Material Safety Data Sheet (MSDS) for HU-210 can provide information about its properties and safety considerations.
- Caffeine Toxicity Factors: Factors affecting caffeine toxicity were reviewed in a 1967 study.
- Toxicity of Cannabinoids: A 1973 study compared the acute oral toxicity of cannabinoids in rats, dogs, and monkeys.
- Delta-8-Tetrahydrocannabinol: Information about Delta-8-THC, a related compound, can be found in the ChemIDplus database.
- International Drug Control Conventions: The United Nations Office on Drugs and Crime provides details on international drug control conventions.
- Synthetic Cannabinoids: Information about synthetic cannabinoids, including HU-210, is available from the New Zealand Drug Foundation.
- Schedule I Classification: The U.S. Drug Enforcement Administration’s Office of Diversion Control lists HU-210 as a Schedule I controlled substance.
- Controlled Substance in Alabama: HU-210 is classified as a Schedule I controlled substance in Alabama, as per Senate Bill 333 from 2014.
- Florida’s Controlled Substances Act: Florida’s Controlled Substances Act designates HU-210 as a Schedule I hallucinogenic substance.
- Vermont’s Regulated Drug Rule: Vermont regulates HU-210 as a “Hallucinogenic Drug” under its regulated drug rule.