Summary
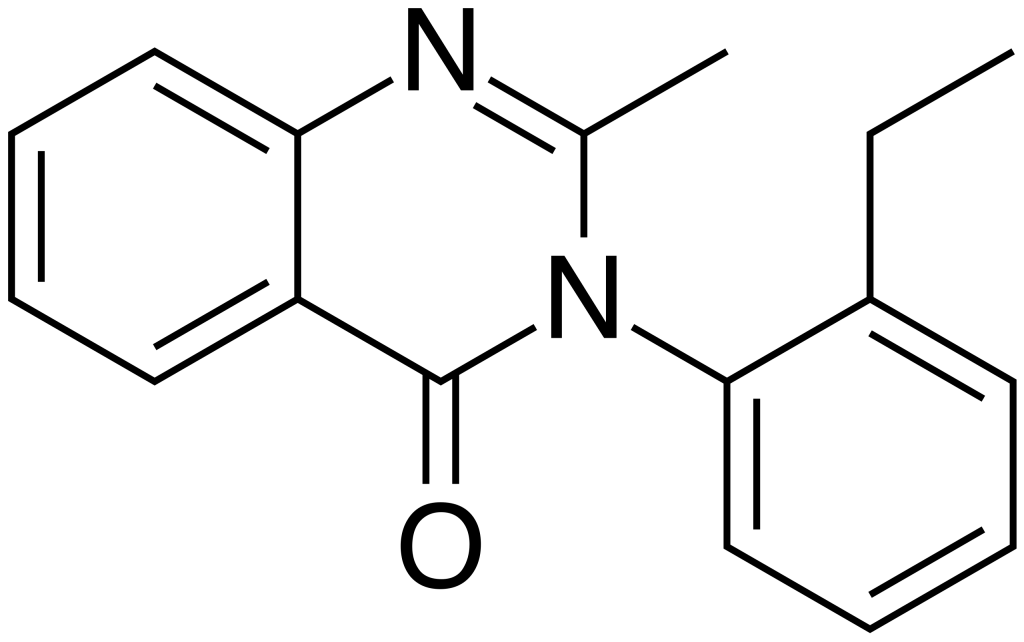
Methaqualone, known by trade names such as Aolan, Athinazone, and Ethinazone, belongs to the quinazolinone class of compounds. This substance is a GABAergic agent and serves as an analog of methaqualone. It was initially developed during the 1960s and found its primary market in France and a few other European countries. Ethaqualone exhibits various properties, including sedative, hypnotic, muscle relaxant, and central nervous system depressant effects. These effects stem from its ability to act as an agonist at the β-subtype of the GABAA receptor, making it useful in the treatment of insomnia.
Typical dosages and effects of etaqualone are reported to be similar to methaqualone, albeit with a shorter duration of action and slightly reduced potency. Standard dosages range from 50 to 500 mg of etaqualone, depending on the desired effects. In the past, pharmaceutical formulations of Ethinazone were available in the form of 350 mg tablets. It is believed that etaqualone operates in a manner akin to barbiturates and benzodiazepines by enhancing the sensitivity of GABAA receptors, although this requires further citation for validation.
Recreational use of etaqualone is associated with euphoria, relaxation, increased sociability, heightened sexuality, impaired short-term memory, and diminished coordination. Combining it with other depressant substances can lead to a potentiated effect and potential overdose. Tolerance developed from the use of benzodiazepines or alcohol may also reduce the effects of etaqualone.
Ethaqualone can exist in two primary forms: a free base, which is insoluble in water but dissolves in alcohol and nonpolar solvents, or a water-soluble hydrochloride salt, which is approximately 85% as potent as the freebase when measured by weight.
The most common method of etaqualone administration is oral ingestion. However, there have been reports of individuals snorting the hydrochloride salt or smoking the free base for a more immediate effect.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 7432-25-9 |
|---|---|
| PubChem CID | 23914 |
| ChemSpider | 22357 |
| UNII | HFS3HB32J7 |
| ChEMBL | ChEMBL2104633 |
| CompTox Dashboard (EPA) | DTXSID60225333 |
| Chemical and physical data | |
| Formula | C17H16N2O |
| Molar mass | 264.322 g·mol−1 |
FAQ
1. What is Ethaqualone?
Ethaqualone is a quinazolinone-class compound and a GABAergic agent. It is an analog of Methaqualone, known by various trade names, and was developed in the 1960s. It possesses sedative, hypnotic, muscle relaxant, and central nervous system depressant properties.
2. How is Ethaqualone different from Methaqualone?
Methaqualone is structurally similar to Methaqualone but tends to have a shorter duration of action and is slightly less potent. Both substances share similar properties, such as sedation and muscle relaxation, but Ethaqualone is known for its distinct characteristics.
3. What is Ethaqualone used for?
Methaqualone was primarily used for the treatment of insomnia due to its sedative and hypnotic effects. It acts as an agonist at the β-subtype of the GABAA receptor, resulting in its therapeutic potential for sleep-related disorders.
4. What are the typical dosages for Ethaqualone?
Typical dosages of Ethaqualone range from 50 to 500 mg, depending on the desired effects. Old pharmaceutical formulations of Ethinazone, a form of Ethaqualone, were available in 350 mg tablets.
5. What are the recreational effects of Ethaqualone?
Recreational use of Ethaqualone may result in effects such as euphoria, relaxation, increased sociability, heightened sexuality, impairment of short-term memory, and reduced coordination.
6. What are the risks associated with Ethaqualone use?
Combining Ethaqualone with other depressant substances can have a potentiating effect and increase the risk of overdose. Additionally, tolerance developed from the use of benzodiazepines or alcohol can reduce the effectiveness of Methaqualone.
7. In what forms is Ethaqualone available?
Ethaqualone can exist as a free base, which is insoluble in water but dissolves in alcohol and nonpolar solvents. It can also be found as a water-soluble hydrochloride salt, which is about 85% as potent as the free when measured by weight.
8. What are the different methods of administration for Ethaqualone?
The most common route of administration for Ethaqualone is oral ingestion. However, there have been reports of individuals snorting the hydrochloride salt or smoking the free base for a more immediate effect.
9. Is Ethaqualone safe for use?
The safety of Ethaqualone use is a subject of concern, especially in recreational contexts. It is essential to be aware of potential risks and to use it only as prescribed by a medical professional if it is available for therapeutic use.
10. Is Ethaqualone legal in my country?
The legal status of Ethaqualone varies by country and jurisdiction. It is essential to research and understand the specific regulations and laws regarding its possession and use in your region before considering any form of consumption.
References
- In November 1967, Pflegel P and Wagner G conducted polarography research on 2-methyl-3-(2-methylphenyl)-3,4-dihydroquinazolinone-(4) (methaqualone, Dormutil) and 2-methyl-3-(2-ethylphenyl-3,4-dihydroquinazolinone-(4) (ethinazone, Aolan). This research delved into the polarography of heterocyclic compounds. The findings were published in Die Pharmazie (in German), Volume 22, Issue 11, with pages 643–650. The publication can be referenced with PMID: 5619478.
- The GB patent 936902, titled “Quinazolinone Derivatives,” was issued on September 18, 1963. This patent was assigned to Beiersdorf.
- In January 1969, Parmar SS, Kishor K, Seth PK, and Arora RC investigated the role of alkyl substitution in 2,3-disubstituted and 3-substituted 4-quinazolones concerning the inhibition of pyruvic acid oxidation. Their findings were published in the Journal of Medicinal Chemistry, Volume 12, Issue 1, on pages 138–141. The publication can be located with the DOI: 10.1021/jm00301a035 and the PMID: 4303122.