Summary
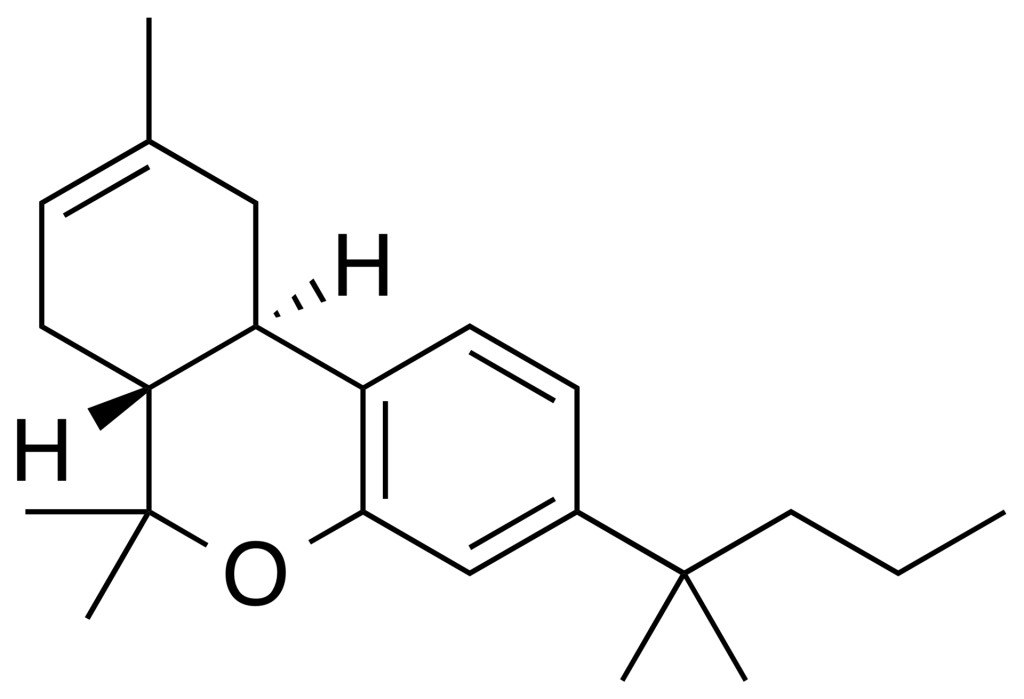
JWH-133, also known as Dimethylbutyl-deoxy-Delta-8-THC, stands out as a potent and highly selective agonist of the CB2 receptor, exhibiting a remarkable Ki value of 3.4nM and an impressive selectivity of approximately 200 times for CB2 over CB1 receptors. This compound owes its name to the pioneering researcher John W. Huffman.
In scientific literature, JWH-133 has occasionally been mistaken for other analogs of Delta-8-THC. Confusion has arisen with compounds such as Dimethylpentyl-Delta-8-THC and Dimethylbutyl-Delta-8-THC, even extending to errors involving the chemical nomenclature itself. It has also been wrongly associated with the Delta-9 isomer.
Structurally, the primary distinction between JWH-133 and dimethylbutyl-D8-THC lies in the absence of a hydroxy group on the phenol structure at the C1 position of the A ring in JWH-133, resulting in a phenyl group. This modification aligns with the consensus that the removal of the hydroxy group from the phenol structure in classical cannabinoid benzopyran, like THC, leads to significantly reduced CB1 activity and heightened CB2 activity.
JWH-133, alongside compounds like WIN 55,212-2 and HU-210, has exhibited the potential to mitigate inflammation induced by Amyloid beta proteins, which are implicated in Alzheimer’s disease. It not only helps prevent cognitive impairment and the loss of neuronal markers but also exerts its anti-inflammatory effects by acting as an agonist at the CB2 receptor. This action inhibits microglial activation, a key trigger for inflammation, and effectively eliminates neurotoxicity associated with microglia activation in rat models.
Promisingly, preliminary data from a 2010 study in Madrid has suggested potential anti-cancer properties associated with JWH-133.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 259869-55-1 |
|---|---|
| PubChem CID | 6918505 |
| IUPHAR/BPS | 747 |
| ChemSpider | 5293702 |
| UNII | TDG8048RDA |
| ChEBI | CHEBI:146243 |
| ChEMBL | ChEMBL371214 |
| CompTox Dashboard (EPA) | DTXSID30426077 |
| Chemical and physical data | |
| Formula | C22H32O |
| Molar mass | 312.497 g·mol−1 |
Legal Status
JWH-133 is not explicitly classified as a controlled substance under the United States Controlled Substance Act. However, it could be deemed an analog of THC under the Federal Analogue Act if it is marketed for human consumption.
FAQ
1. What is JWH-133?
JWH-133 is a compound known as Dimethylbutyl-deoxy-Delta-8-THC, which acts as a potent and selective agonist of the CB2 receptor in the endocannabinoid system.
2. Who discovered JWH-133, and why is it named after John W. Huffman?
John W. Huffman, a renowned researcher in synthetic cannabinoids, discovered JWH-133. It bears his name as a tribute to his pioneering work.
3. What makes JWH-133 unique in terms of cannabinoid receptors?
JWH-133 is distinguished by its extraordinary selectivity for the CB2 receptor, with a Ki value of 3.4nM, making it highly potent and selective for CB2 over CB1 receptors.
4. Why has JWH-133 been confused with other compounds in scientific literature?
JWH-133 has been occasionally confused with other analogs of Delta-8-THC, such as Dimethylpentyl-Delta-8-THC and Dimethylbutyl-Delta-8-THC, along with errors in its chemical nomenclature. This confusion arises due to structural similarities.
5. What is the structural difference between JWH-133 and dimethylbutyl-D8-THC?
The primary structural difference is the absence of a hydroxy group on the phenol structure at the C1 position of the A ring in JWH-133. This modification results in a phenyl group influencing its receptor activity.
6. What are the potential medical applications of JWH-133?
JWH-133, in conjunction with other compounds like WIN 55,212-2 and HU-210, has shown promise in mitigating inflammation caused by Amyloid beta proteins associated with Alzheimer’s disease. It can also prevent cognitive impairment and loss of neuronal markers through its agonist action at the CB2 receptor.
7. How does JWH-133 exert its anti-inflammatory effects?
JWH-133 acts as an agonist at the CB2 receptor, which helps prevent microglial activation, a key trigger for inflammation. This, in turn, eliminates neurotoxicity linked to microglia activation in experimental models.
8. Is there any potential link between JWH-133 and anti-cancer properties?
Preliminary data from a 2010 study in Madrid has hinted at potential anti-cancer properties associated with JWH-133, although further research is needed to establish its efficacy and safety in this regard.
9. Is JWH-133 legally available for use or research?
The legal status of JWH-133 may vary by jurisdiction. Still, it’s important to note that synthetic cannabinoids often face regulatory restrictions due to their potential risks and the need for further research.
10. Are there potential risks or side effects associated with JWH-133 use?
The use of synthetic cannabinoids, including JWH-133, can be associated with unpredictable and potentially harmful effects. Its safety and legal status should be carefully considered, and consulting with a healthcare professional is advisable.
References
- Bow EW and Rimoldi JM, in a paper published on June 28, 2016, delved into the intricate “Structure-Function Relationships of Classical Cannabinoids,” exploring the modulation of CB1 and CB2 receptors. Their work, featured in “Perspectives in Medicinal Chemistry,” offers valuable insights into this area. [DOI: 10.4137/PMC.S32171] [PMC: 4927043] [PMID: 27398024]
- A compound known as “(6AR,10AR)-3-(1,1-Dimethylbutyl)-6A,7,10,10A-tetrahydro-6,6,9-trimethyl-6H-dibenzo[B,D]pyran” was the subject of investigation. This compound is a part of the evolving cannabinoid research landscape.
- Huffman JW and his team, in a study from December 1999, focused on “3-(1′,1′-Dimethylbutyl)-1-deoxy-delta8-THC and related compounds.” They explored the synthesis of selective ligands for the CB2 receptor, contributing to our understanding of cannabinoid pharmacology. Published in “Bioorganic & Medicinal Chemistry,” Volume 7, Issue 12. [DOI: 10.1016/S0968-0896(99)00219-9] [PMID: 10658595]
- In April 2003, Huffman JW, Miller JR, and their colleagues investigated “Structure-activity relationships for 1′,1′-dimethylalkyl-Delta8-tetrahydrocannabinols.” This research, found in “Bioorganic & Medicinal Chemistry,” Volume 11, Issue 7, explored the relationships between chemical structure and biological activity. [DOI: 10.1016/s0968-0896(02)00649-1] [PMID: 12628666]
- Caffarel MM, Andradas C, and their team made notable contributions in July 2010 with their research on how “Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition.” Published in “Molecular Cancer,” their work sheds light on the potential anti-cancer properties of cannabinoids. [DOI: 10.1186/1476-4598-9-196] [PMC: 2917429] [PMID: 20649976]
- Additionally, the NORML organization reported on August 5, 2010, that a “Marijuana Compound Halts Breast Cancer Tumor Growth,” highlighting the potential medical significance of cannabinoids in cancer research.
- For further details and access to these publications, you can refer to the “Federal Register :: Request Access.”