Contents
Summary
Mazindol, available under brand names such as Mazanor and Sanorex, is classified as a stimulant medication primarily employed for appetite suppression. This medication was originally formulated by Sandoz-Wander during the 1960s.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 22232-71-9 |
|---|---|
| PubChem CID | 4020 |
| IUPHAR/BPS | 4797 |
| DrugBank | DB00579 |
| ChemSpider | 3880 |
| UNII | C56709M5NH |
| KEGG | D00367 |
| ChEMBL | ChEMBL781 |
| CompTox Dashboard (EPA) | DTXSID1023237 |
| ECHA InfoCard | 100.040.764 |
| Chemical and physical data | |
| Formula | C16H13ClN2O |
| Molar mass | 284.74 g·mol−1 |
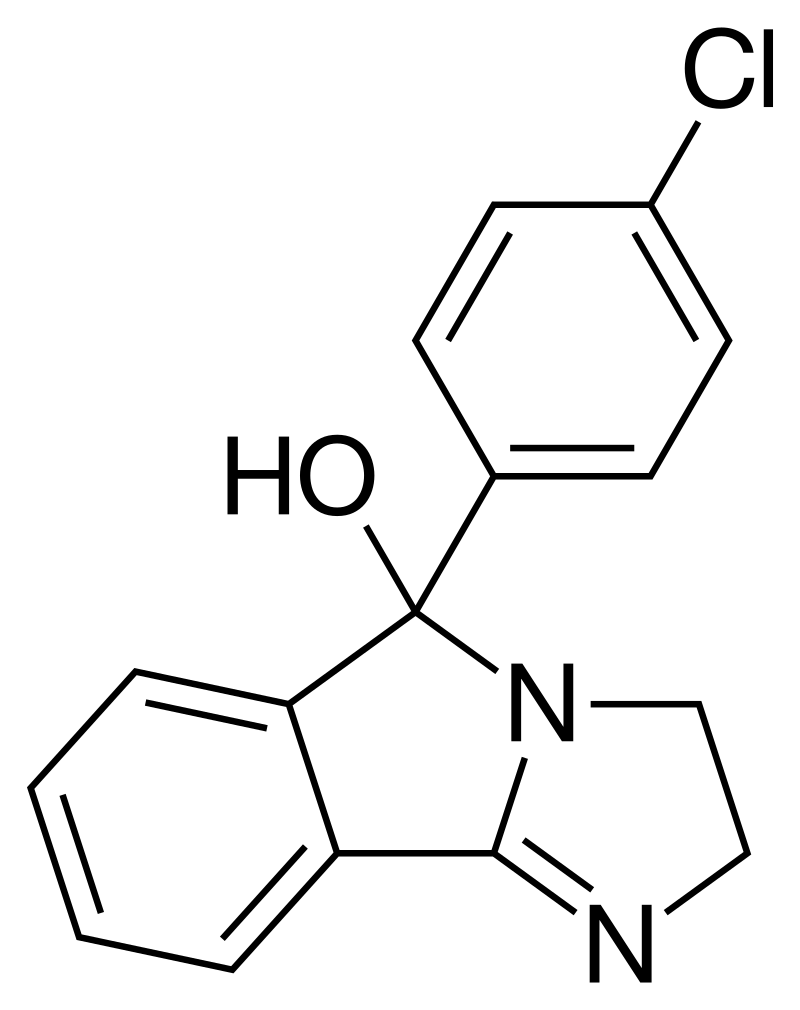
Medical uses
Mazindol is utilized in the short-term, typically lasting a few weeks, as part of a comprehensive obesity management strategy. This strategy combines caloric restriction, exercise, and behavioral modifications. It is prescribed for individuals with a body mass index (BMI) exceeding 30 or for those with a BMI over 27 when accompanied by risk factors like hypertension, diabetes, or hyperlipidemia. Currently, Mazindol is not available as a commercially marketed prescription drug regulated by the FDA for obesity treatment.
A Swiss study is actively investigating its potential effectiveness in addressing ADHD.
Furthermore, Mazindol holds patents for various applications, including the treatment of schizophrenia, reducing cravings associated with cocaine use, and managing neurobehavioral disorders.
Pharmacology
Mazindol belongs to the class of sympathomimetic amines, sharing similarities with amphetamine. It exerts its effects by stimulating the central nervous system, resulting in increased heart rate and blood pressure while concurrently reducing appetite. These sympathomimetic anoretics, often employed in the short-term management of obesity, tend to exhibit diminishing appetite-suppressing effects after a few weeks of use. Consequently, they are most beneficial during the initial stages of a weight loss program.
While the exact mechanism underlying sympathomimetics’ action in obesity treatment remains incompletely understood, these medications exhibit pharmacological actions akin to amphetamines. Like other sympathomimetic appetite suppressants, mazindol is believed to function as a reuptake inhibitor of norepinephrine. Additionally, it inhibits the reuptake of dopamine and serotonin.
The recommended dosage for mazindol varies from 2 mg daily for 90 days in patients with a body weight exceeding 40 kg but less than 50 kg and 4 mg per day in patients with a body weight exceeding 50 kg. This daily dosage is typically divided into two separate doses with a 12-hour interval between each administration.
Overdose
An overdose of mazindol may manifest with a range of symptoms, such as restlessness, tremors, accelerated breathing, confusion, hallucinations, heightened anxiety, aggressive behavior, nausea, vomiting, diarrhea, irregular heart rhythm, and seizures.
Analogues
A derivative of mazindol was identified, purportedly possessing lower toxicity compared to its parent compound. This derivative is synthesized using Chemrat, also known as Pindone, as its precursor.
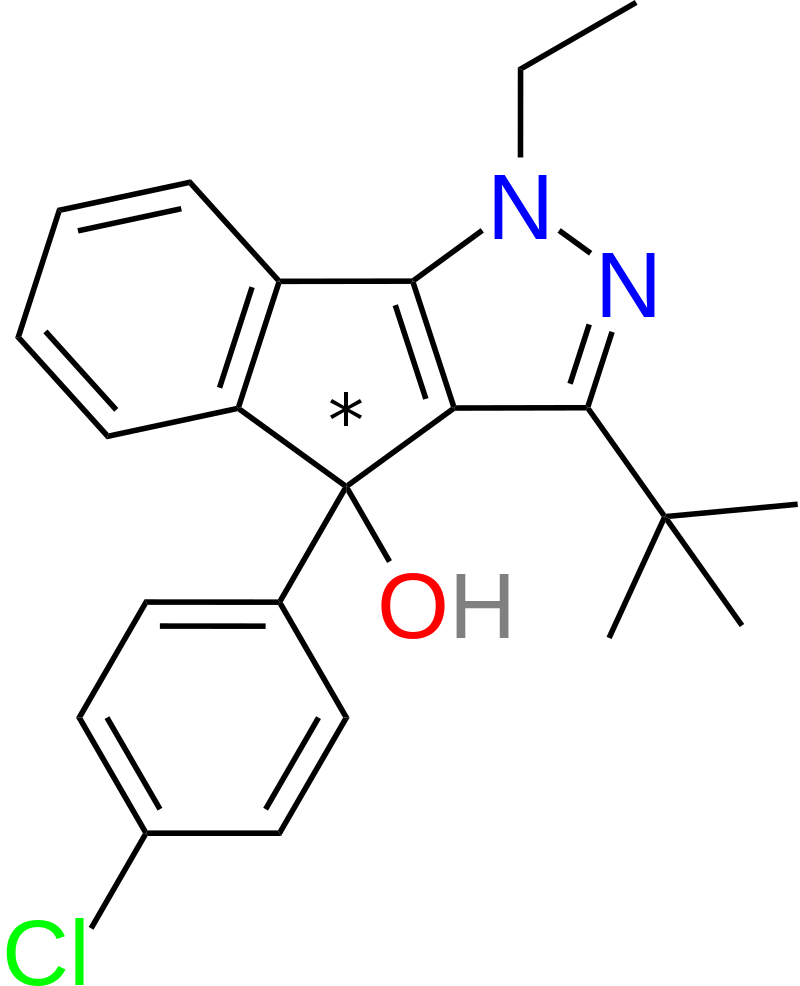
QSAR Dialog
Based on available QSAR (Quantitative Structure-Activity Relationship) data, the following patterns have been observed:
- Removing the tertiary alcohol group in mazindol, a process known as desoxylation, enhances its binding to DAT (Dopamine Transporter) and SERT (Serotonin Transporter) receptors without significantly affecting NET (Norepinephrine Transporter) affinity. This modified compound is often referred to as “Mazindane.”
- Eliminating the p-chlorine atom attached to the phenyl ring in mazindol results in increased NET affinity but significantly reduces affinity for DAT and SERT.
- Enlarging the imidazoline ring system in mazindol to its corresponding six-membered counterpart substantially boosts DAT affinity by approximately tenfold.
- Replacing the phenyl group with a naphthyl ring system leads to a significant (~50-fold) increase in SERT affinity, with no substantial decreases in NET or DAT affinities.
- Introducing halogen atoms (e.g., chlorine or fluorine) at the 3′ and 4′ positions of the phenyl ring in mazindol enhances its potency in binding to NET, DAT, and SERT receptors.
- Fluorination of the 7′ position on the tricyclic phenyl ring results in approximately a twofold increase in binding affinity to DAT.
| Compound | S. Singh’s alphanumeric assignation (name) | R | R′ | R′′ | IC50 (nM) (Inhibition of [3H]WIN 35428 binding) | IC50 (nM) (Inhibition of [3H]DA uptake) | Selectivity uptake/binding |
|---|---|---|---|---|---|---|---|
| (cocaine) | 89.1 ± 8 | 208 ± 12 | 2.3 | ||||
| (mazindol) | H | H | 4′-Cl | 8.1 ± 1.2 | 8.4 ± 1.3 | 1.0 | |
| 384a | H | H | H | 66.0 ± 8.9 | 124 ± 37 | 1.9 | |
| 384b | H | H | 4′-F | 13.3 ± 1.8 | 25.4 ± 2.7 | 1.9 | |
| 384c | H | 7-F | H | 29.7 ± 7.0 | 78 ± 46 | 2.6 | |
| 384d | H | H | 2′-Cl | 294 ± 6 | 770 ± 159 | 2.6 | |
| 384e | H | H | 3′-Cl | 4.3 ± 0.4 | 9.2 ± 5.3 | 2.1 | |
| 384f | CH3 | H | 4′-Cl | 50.4 ± 5.5 | 106 ± 5.6 | 2.1 | |
| 384g | H | 6-Cl | H | 57.2 ± 8.3 | 58 ± 6.4 | 1.0 | |
| 384h | H | 7-Cl | H | 85.4 ± 14 | 55.17 | 0.6 | |
| 384i | H | 7-F | 4′-Cl | 6.5 ± 1.2 | 15 ± 9 | 2.3 | |
| 384j | H | 7-Cl | 4′-F | 52.8 ± 8.7 | 53 ± 18 | 1.0 | |
| 384k | H | H | 2′,4′-Cl2 | 76.5 ± 1.11 | 92 ± 19 | 1.2 | |
| 384l | H | H | 3′,4′-Cl2 | 2.5 ± 0.5 | 1.4 ± 1.6 | 0.6 | |
| 384m | H | 7,8-Cl2 | 4′-Cl | 13.6 ± 1.5 | |||
| 384n | H | H | 2′-Br | 1340 ± 179 | |||
| 384o | H | H | 4′-Br | 2.6 ± 1.5 | 8.6 ± 3.5 | 3.3 | |
| 384p | H | H | 4′-I | 17.2 ± 0.9 | 14 ± 6.4 | 0.8 |
Chemistry
Mazindol displays pH-dependent keto-enol tautomerization, as illustrated by the enol form on the left and the keto form on the right. In neutral conditions, mazindol predominantly exists in its tricyclic (-ol) form, while in acidic environments, it undergoes protonation, transforming into the benzophenone tautomer. Research through Quantitative Structure-Activity Relationship (QSAR) studies has suggested that mazindol’s capacity to inhibit the reuptake of norepinephrine (NE) and dopamine (DA) may be influenced by the protonated (benzophenone) tautomer.
Synthesis
The precursor for mazindol is detailed in the synthesis of Chlortalidone.
- Thieme Synthesis:
- Patents:
The synthesis of mazindol initiates with the reaction of a substituted benzoylbenzoic acid (1) with ethylenediamine. The resulting product, 3, can be explained as an aminal originating from the initially formed monoamide 2. This compound is subsequently subjected to reduction using LiAlH4, followed by air oxidation without intermediate isolation. Reduction likely progresses to form the mixed aminal/carbinolamine 4, with equilibrium favoring the alternate aminal 5 due to the greater stability of aldehyde aminals over their corresponding ketone counterparts. Air oxidation of the tetrahydroimidazole transforms it into imidazoline, effectively removing 5 from the equilibrium. This process ultimately yields the anorectic agent mazindol (6). Homomazindol, the homolog with a six-membered ring A, can be synthesized by substituting 1,2-diaminoethane with 1,3-diaminopropane.
An alternative synthesis method is as follows:
- Mazindol synthesis (alternative):
- 2-Phenyl-2-Imidazoline
- Methyl 4-Chlorobenzoate
FAQ
1. What is Mazindol?
- Mazindol is a medication classified as a sympathomimetic amine. It is used primarily as an appetite suppressant and is employed in the short-term treatment of obesity.
2. How does Mazindol work?
- Mazindol stimulates the central nervous system, leading to increased heart rate and blood pressure while simultaneously reducing appetite. This mechanism helps individuals control their food intake and supports weight loss.
3. What are the recommended uses for Mazindol?
- Mazindol is typically prescribed for individuals with a body mass index (BMI) greater than 30 as part of a comprehensive weight management program. It may also be considered for those with a BMI exceeding 27 in the presence of certain risk factors like hypertension, diabetes, or hyperlipidemia. Mazindol is meant for short-term use in conjunction with calorie restriction, exercise, and behavioral modifications.
4. Are there any potential side effects of Mazindol?
- Yes, Mazindol may have side effects. Common side effects can include restlessness, tremors, rapid breathing, confusion, hallucinations, panic, aggressiveness, nausea, vomiting, diarrhea, irregular heartbeat, and seizures. It’s crucial to discuss any concerns or side effects with your healthcare provider.
5. What is the tautomeric behavior of Mazindol?
- Mazindol can exist in different tautomeric forms, primarily keto and enol. Its tautomeric state can change depending on the pH of the environment, which can have implications for its biological activity.
6. How is Mazindol synthesized?
- The synthesis of Mazindol typically involves chemical reactions starting with specific precursor compounds. These reactions can vary depending on the specific synthesis method employed.
7. Is Mazindol currently available as a prescription medication for obesity treatment?
- As of my last knowledge update in September 2021, Mazindol may not be commercially marketed or FDA-regulated as a prescription agent for obesity treatment. However, regulatory statuses can change over time, so it’s essential to consult with a healthcare professional for the most up-to-date information on its availability.
8. Are there alternative medications for obesity treatment?
- Yes, there are other medications and approaches available for obesity treatment. These can include different appetite suppressants, weight loss medications, and lifestyle changes such as diet and exercise. Your healthcare provider can help determine the most suitable approach for your specific needs.
9. Can Mazindol be used to treat conditions other than obesity?
- Research has explored potential alternative uses for Mazindol, such as its efficacy in treating ADHD and other neurobehavioral disorders. However, its primary approved use remains as an appetite suppressant in obesity management.
10. Is Mazindol safe for everyone?
- Mazindol, like any medication, has specific safety considerations and potential contraindications. It should only be used under the guidance and supervision of a qualified healthcare provider who can assess its suitability for an individual’s specific medical history and needs.
References
- Anvisa’s Resolution on Controlled Substances: Anvisa (Agência Nacional de Vigilância Sanitária) issued Resolution No. 784, which pertains to lists of narcotic, psychotropic, precursor, and other substances under special control. This resolution, in Brazilian Portuguese, was published on March 31, 2023, in the Diário Oficial da União and is archived from the original as of August 3, 2023.
- Pharmacological Profile of Mazindol: In 1978, a study titled “Pharmacology and biochemical profile of a new anorectic drug: mazindol” was conducted. This research delved into the pharmacological and biochemical characteristics of mazindol as an anorectic drug.
- US Patent for 1H-Isoindole Intermediates: A United States patent (US granted 3597445) issued on August 3, 1971, to Houlihan WJ and Eberle MK pertains to 1H-Isoindole intermediates and is assigned to Sandoz AG.
- NLS Pharma’s ADHD Drug: A Reuters article from May 31, 2017, reports on the success of Swiss biotech company NLS Pharma’s ADHD drug in a mid-stage study.
- Yale University Patent: John P. Seibyl holds U.S. Patent 5,447,948 (1995), related to certain pharmaceutical applications, assigned to Yale University.
- US Patent by Stephen P. Berger: U.S. Patent 5,217,987 (1993) is credited to Stephen P. Berger and pertains to specific pharmaceutical inventions.
- WO2009155139 Patent by Afecta Pharmaceuticals Inc: A patent with the identifier WO2009155139 is attributed to Afecta Pharmaceuticals Inc and relates to certain pharmaceutical developments.
- Research on Amphetamine-Type Stimulants: Research conducted in January 2001, titled “Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin,” explores the release of neurotransmitters by amphetamine-type stimulants.
- Analog Studies of Mazindol: A study from 1975, titled “Analogs of the Anorexic Mazindol,” investigates analogs of mazindol and their potential applications.
- Chemistry of Cocaine Antagonists: A research paper from March 2000 discusses the chemistry, design, and structure-activity relationship of cocaine antagonists.
- Inhibitors of Cocaine Binding Site: Several patents (e.g., U.S. Patent 5,217,987 and U.S. Patent 5,447,948) explore the development of compounds as potential inhibitors of the cocaine binding site at the dopamine transporter.
- Mazindane Pro-Drug Assessment: Research from 2003 assesses mazindane, a pro-drug form of mazindol, in assays used to define cocaine treatment agents.
- Molecular Geometry of Uptake Inhibitors: A study from December 1976 examines the molecular geometry of inhibitors of the uptake of catecholamines and serotonin in rat brain synaptosomal preparations.
- Anorectic Agents Research: Research from February 1975 investigates 5-aryl-2,3-dihydro-5H-imidazo[2,1-a]isoindol-5-ols, a novel class of anorectic agents.
- German Patents: Several German patents (e.g., DE granted 1814540 and DE granted 1930488) relate to the development and synthesis of various compounds, including imidazoisoindole derivatives.
- US Patent on Midazolinyl Phenyl Carbonyl Acid Addition Salts: A U.S. patent (US granted 3763178) issued on October 2, 1973, pertains to midazolinyl phenyl carbonyl acid addition salts and related compounds.
- Optimizing ADHD Treatment: A publication from December 2016 discusses strategies for optimizing outcomes in ADHD treatment, including novel delivery systems.