Summary
MN-25 (UR-12) is a pharmaceutical creation credited to Bristol-Myers Squibb. This drug primarily functions as a relatively selective agonist, predominantly stimulating peripheral cannabinoid receptors. Its affinity for CB2 receptors is moderate, featuring a Ki value of 11 nM. Notably, it displays significantly lower affinity, approximately 22 times lower, for the psychoactive CB1 receptors, with a Ki of 245 nM. An interesting reversal of the affinity ratio occurs in the case of its indole 2-methyl derivative, where it exhibits a Ki of 8 nM at CB1 and 29 nM at CB2, contrary to the usual trend of 2-methyl products having enhanced selectivity for CB2.
From a chemical perspective, MN-25 shares close structural relations with another synthetic cannabinoid, Org 28611, belonging to the indole-3-carboxamide family. However, MN-25 diverges by featuring a different cycloalkyl substitution on the carboxamide and replacing the cyclohexyl methyl group with morpholinyl ethyl, akin to JWH-200 or A-796,260. These early compounds, including MN-25, have subsequently paved the way for developing various related indole-3-carboxamide cannabinoid ligands.
| Identifiers | |
|---|---|
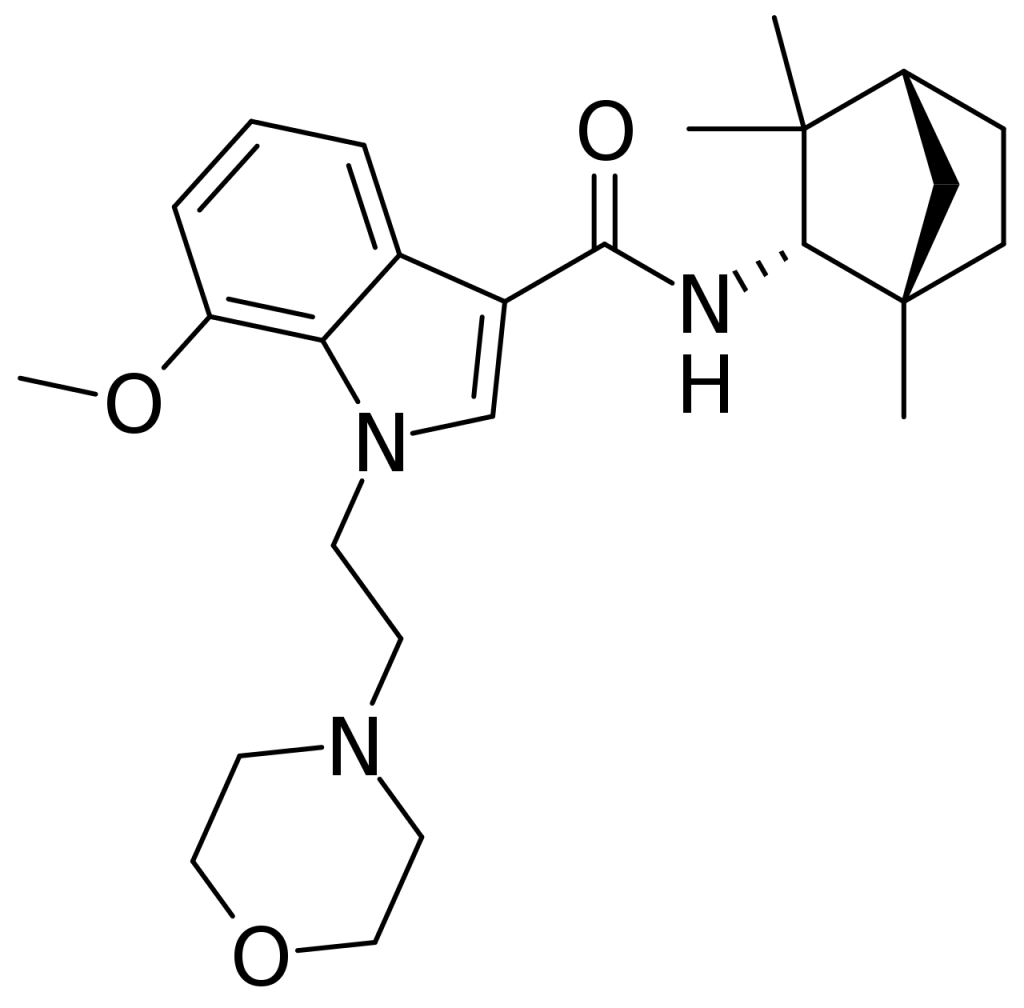
| IUPAC name | |
| CAS Number | 501926-82-5 2-methyl derivative: 501927-29-3 |
|---|---|
| PubChem CID | 71308243 |
| ChemSpider | 26286811 |
| UNII | 8WXU5YRE25 |
| ChEMBL | ChEMBL3234671 |
| Chemical and physical data | |
| Formula | C26H37N3O3 |
| Molar mass | 439.600 g·mol−1 |
FAQ
1. What is MN-25 (UR-12)?
MN-25, also known as UR-12, is a synthetic drug developed by Bristol-Myers Squibb. It acts as an agonist of peripheral cannabinoid receptors.
2. How does MN-25 affect cannabinoid receptors?
MN-25 exhibits moderate affinity for CB2 receptors with a Ki value of 11 nM. However, it has a considerably lower affinity for CB1 receptors, with a Ki value of 245 nM, making it less psychoactive.
3. What is the significance of the affinity reversal in the 2-methyl derivative of MN-25?
The 2-methyl derivative of MN-25 demonstrates a reversal in affinity ratios, with a Ki value of 8 nM at CB1 and 29 nM at CB2. This contrasts with the typical trend where 2-methyl derivatives exhibit increased selectivity for CB2 receptors.
4. How is MN-25 related to other synthetic cannabinoids?
Chemically, MN-25 is closely related to another indole-3-carboxamide synthetic cannabinoid called Org 28611. It features different cycloalkyl and carboxamide substitutions, with the cyclohexyl methyl group replaced by morpholinyl ethyl, similar to compounds like JWH-200 and A-796,260. These early compounds have paved the way for the development of related indole-3-carboxamide cannabinoid ligands.
5. Is MN-25 still in use or production?
The availability and legal status of MN-25 may vary by region and over time. It’s important to check local regulations and laws to determine its current level.
6. Are there any health risks associated with MN-25 use?
As with all synthetic drugs, there are potential health risks associated with the use of MN-25. It’s essential to be cautious and informed about the possible effects and safety concerns.
7. Where can I find more information about MN-25 and related compounds?
You can access additional information about MN-25 and related synthetic cannabinoids through scientific literature, research publications, and reputable sources focused on substance abuse prevention and awareness. Staying informed is vital for understanding the potential risks associated with these substances.
References
- WO application 0158869, authored by Leftheris K, Zhao R, Chen BC, Kiener P, Wu H, Pandit CR, Wrobleski S, Chen P, Hynes J, Longphre M, Norris DJ, Spergel S, Tokarski J, titled “Cannabinoid Receptor Modulators, Their Processes of Preparation, and use of Cannabinoid Receptor Modulators for Treating Respiratory and Non-Respiratory Diseases,” was published on August 16, 2001, and is assigned to Bristol-Myers Squibb.
- In December 2009, Zhao R, Wang B, Wu H, Hynes J, Leftheris K, Balasubramanian B, Barrish JC, and Chen BC improved the procedure for preparing 7-methoxy-2-methyl-1-(2-morpholinoethyl)-1H-indole-3-carboxylic acid, a key intermediate in the synthesis of novel 3-amidoindole and indolopyridone cannabinoid ligands. This research is documented in Arkivoc.
- In September 2002, Hynes J, Leftheris K, Wu H, Pandit C, Chen P, Norris DJ, and others explored “C-3 Amido-indole cannabinoid receptor modulators” in Bioorganic & Medicinal Chemistry Letters.
- Wrobleski ST, Chen P, Hynes J, Lin S, Norris DJ, Pandit CR, Spergel S, Wu H, Tokarski JS, Chen X, and others conducted research on the “Rational design and synthesis of an orally active indolopyridone as a novel conformationally constrained cannabinoid ligand possessing antiinflammatory properties” in the Journal of Medicinal Chemistry in May 2003.
- Huffman JW and Padgett LW discussed “Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles, and indenes” in Current Medicinal Chemistry in 2005.
- Manera C, Tuccinardi T, and Martinelli A delved into “Indoles and related compounds as cannabinoid ligands” in Mini Reviews in Medicinal Chemistry in April 2008.
- Adam JM, Cairns J, Caulfield W, Cowley P, Cumming I, Easson M, and others worked on the “Design, synthesis, and structure–activity relationships of indole-3-carboxamides as novel water-soluble cannabinoid CB1 receptor agonists” in MedChemComm in 2010.
- Kiyoi T, York M, Francis S, Edwards D, Walker G, Houghton AK, Cottney JE, Baker J, and Adam JM explored “Design, synthesis, and structure-activity relationship study of conformationally constrained analogs of indole-3-carboxamides as novel CB1 cannabinoid receptor agonists” in Bioorganic & Medicinal Chemistry Letters in August 2010.
- Moir EM, Yoshiizumi K, Cairns J, Cowley P, Ferguson M, Jeremiah F, and others conducted a “Design, synthesis, and structure-activity relationship study of bicyclic piperazine analogs of indole-3-carboxamides as novel cannabinoid CB1 receptor agonists” in Bioorganic & Medicinal Chemistry Letters in December 2010.
- Blaazer AR, Lange JH, van der Neut MA, Mulder A, den Boon FS, Werkman TR, Kruse CG, and Wadman WJ investigated “Novel indole and azaindole (pyrrolopyridine) cannabinoid (CB) receptor agonists: design, synthesis, structure-activity relationships, physicochemical properties, and biological activity” in the European Journal of Medicinal Chemistry in October 2011.