Summary
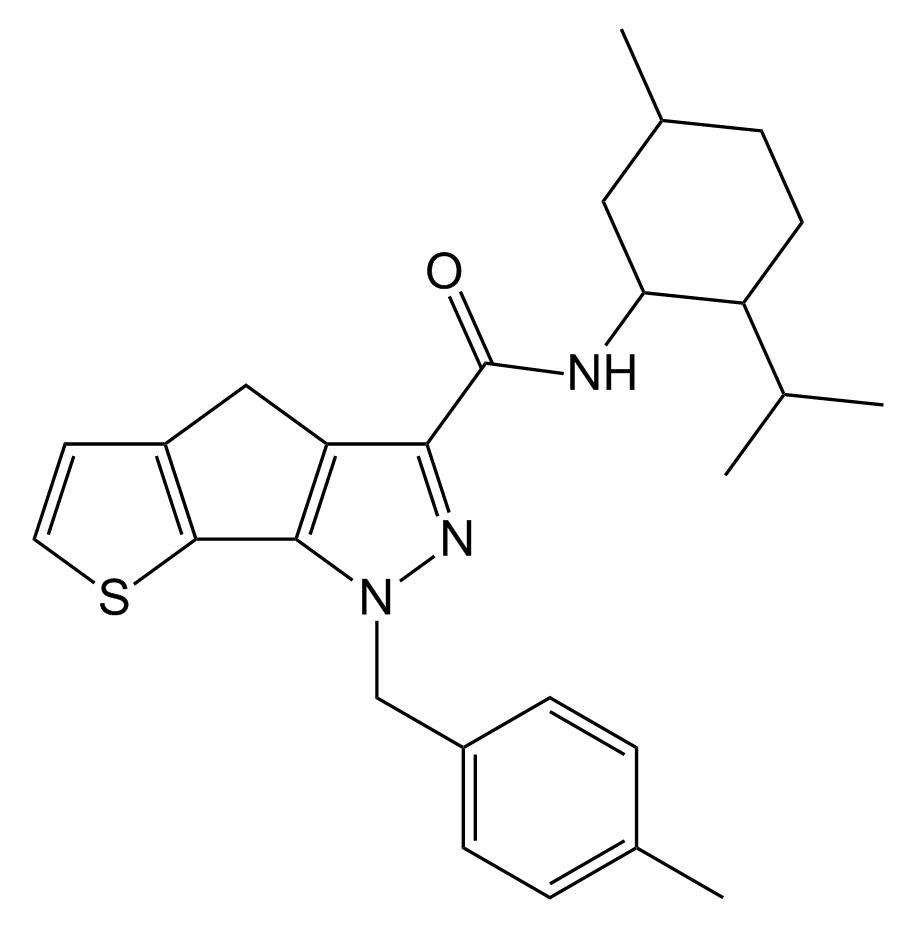
NESS-040C5, designed for glaucoma treatment, is a robust cannabinoid agonist. It demonstrates a notable preference for the CB2 receptor subtype, boasting a remarkable CB2 affinity at 0.4 nanomolar and a selectivity exceeding 25-fold when compared to the closely related CB1 receptor.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1445751-90-5 |
|---|---|
| PubChem CID | 138402821 |
| UNII | P6H2AAY2GE |
| CompTox Dashboard (EPA) | DTXSID201010004 |
| Chemical and physical data | |
| Formula | C27H33N3OS |
| Molar mass | 447.64 g·mol−1 |
FAQ
1. What is NESS-040C5?
NESS-040C5 is a pharmaceutical compound designed for the treatment of glaucoma. It acts as a potent cannabinoid agonist.
2. How does NESS-040C5 function in glaucoma treatment?
NESS-040C5 interacts with cannabinoid receptors, which may have potential applications in glaucoma treatment. It is believed to impact intraocular pressure, a key factor in glaucoma.
3. What is the affinity of NESS-040C5 for the CB2 receptor?
NESS-040C5 exhibits a strong affinity for the CB2 receptor, with a binding affinity of 0.4 nanomolar (nM).
4. Is NESS-040C5 selective for a specific cannabinoid receptor subtype?
Yes, NESS-040C5 displays a notable selectivity for the CB2 receptor subtype. It has a 25-fold selectivity advantage over the closely related CB1 receptor.
5. Can NESS-040C5 be used for conditions other than glaucoma?
While it was developed with glaucoma in mind, researchers may explore potential applications in other medical conditions. However, its use beyond glaucoma is subject to further research and clinical trials.
6. Where can I find more information about NESS-040C5 and its research applications?
You can refer to scientific journals, research articles, and academic resources in cannabinoid research for in-depth information about NESS-040C5 and its potential applications. Additionally, consult with experts and institutions specializing in this area for further guidance.
7. Are there any safety considerations when working with NESS-040C5?
As with any pharmaceutical compound, safety measures should be taken when handling NESS-040C5, especially in a laboratory or clinical setting. Researchers should adhere to established safety guidelines and regulations.
8. Is NESS-040C5 currently available for clinical use?
NESS-040C5 may still be in the experimental or developmental phase and not yet approved for clinical use. Its availability for medical treatments may vary by region and is subject to regulatory approvals.
9. How can I access NESS-040C5 for research or potential medical applications?
Access to NESS-040C5 for research is typically facilitated through collaboration with pharmaceutical companies, research institutions, or organizations involved in cannabinoid-related studies. Legal and ethical regulations should be followed when obtaining and using this compound.
10. Can I participate in clinical trials involving NESS-040C5?
Participation in clinical trials involving NESS-040C5 may be possible, depending on the specific research studies and trial requirements. To explore potential involvement in such trials, you can contact research institutions or medical facilities conducting related studies.
References
- In a groundbreaking development, Paolo Lazzari and his team made significant strides in pharmaceutical compounds as detailed in US Patent 8106218.
- The endocannabinoid system saw new horizons in 2010 when Hanus LO and Mechoulam R explored novel natural and synthetic ligands. Their findings, published in Current Medicinal Chemistry (Volume 17, Issue 14, pages 1341–59), opened doors to potential therapeutic applications. For further insights, you can delve into their research through doi:10.2174/092986710790980096, and the PMID is 20166928.