Summary
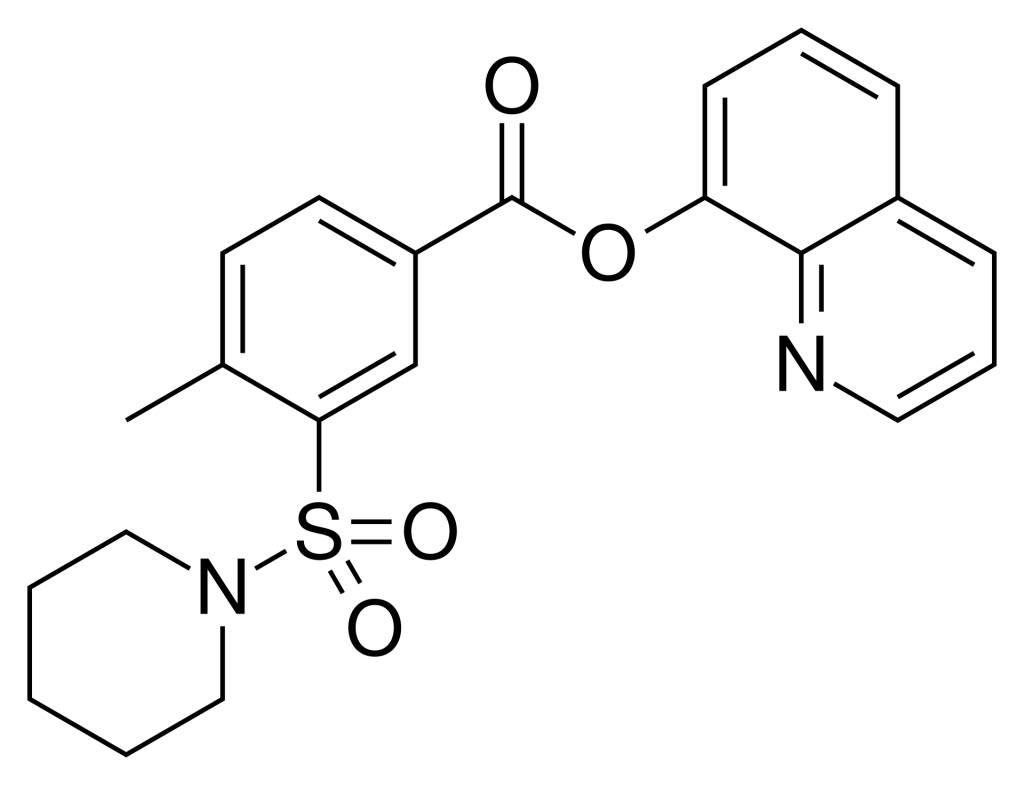
QMPSB, initially identified as an aryl sulfonamide-based synthetic cannabinoid, found its place in the realm of designer drugs.
In 2007, Nathalie Lambeng and her research team unearthed QMPSB, unveiling its role as a robust full agonist of the CB1 and CB2 receptors, boasting impressive Ki values of 3 nM and 4 nM, respectively. Following this discovery, many related derivatives emerged, primarily focusing on enhancing selectivity for the non-psychoactive CB2 receptor. This pursuit continued earlier endeavors involving sulfamoyl benzamide derivatives, culminating in a patent filing 2004.
Interestingly, the distinct quinoline-8-yl ester motif present in QMPSB’s structure paved the way for the discovery of other designer cannabinoids, including notable compounds like PB-22 and BB-22.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 312606-87-4 |
|---|---|
| PubChem CID | 3929482 |
| ChemSpider | 3151524 |
| UNII | 55Q8B94HS5 |
| ChEMBL | ChEMBL245876 |
| Chemical and physical data | |
| Formula | C22H22N2O4S |
| Molar mass | 410.49 g·mol−1 |
FAQ
1. What is QMPSB?
QMPSB is a synthetic cannabinoid with an aryl sulfonamide-based structure commonly associated with designer drugs.
2. Who discovered QMPSB, and when was it first identified?
QMPSB was first discovered by Nathalie Lambeng and colleagues in 2007, marking its initial introduction to the scientific community.
3. How does QMPSB interact with cannabinoid receptors?
QMPSB acts as a full agonist of the CB1 and CB2 receptors, with Ki values of 3 nM and 4 nM, respectively. This suggests its strong binding affinity to these receptors.
4. What was the focus of research related to QMPSB derivatives?
Subsequent research efforts aimed to produce derivatives of QMPSB, with a primary focus on increasing selectivity for the non-psychoactive CB2 receptor. This work sought to enhance the therapeutic potential of related compounds.
5. Is there any patent associated with developing QMPSB or its derivatives?
Yes, a patent related to a series of sulfamoyl benzamide derivatives, which aligns with the development of QMPSB, was filed in 2004. This patent indicates the early stages of research in this field.
6. What are the significant implications of QMPSB’s quinoline-8-yl ester motif?
The unique quinoline-8-yl ester motif within QMPSB’s structure has contributed to discovering other designer cannabinoids, including notable compounds such as PB-22 and BB-22.
7. Is QMPSB legally available or approved for any specific use?
QMPSB and its derivatives are often associated with the designer drug market and may not be legally available for human consumption or medical use. The legal status of such substances can vary by jurisdiction and is subject to regulations.
8. Where can I find more information about QMPSB and related research?
For in-depth information about QMPSB and its research, you can explore scientific journals, articles, and academic resources on synthetic cannabinoids. Additionally, consulting with experts and institutions specializing in this area can provide further insights.
9. Are there safety considerations when dealing with QMPSB or its derivatives?
Handling and researching synthetic cannabinoids like QMPSB should be done with strict adherence to safety protocols, especially in laboratory settings. Researchers should follow established safety guidelines and regulations.
10. Can I participate in studies related to QMPSB or designer cannabinoids?
Participation in research studies involving substances like QMPSB may be possible, depending on the specific research projects and trial requirements. To explore potential involvement in such studies, you can contact research institutions and academic experts in the field.
References
- In March 2016, a team led by Blakey K uncovered a novel synthetic cannabimimetic, 8-quinolinyl 4-methyl-3-(1-piperidinylsulfonyl)benzoate, also known as QMPSB, along with other designer drugs in herbal incense. This pivotal discovery was documented in the Forensic Science International (Volume 260, pages 40–53). To explore their findings in detail, refer to doi:10.1016/j.forsciint.2015.12.001, and the PMID is 26795397.
- In January 2007, Lambeng N, Lebon F, Christophe B, and their team delved into the realm of arylsulfonamides as a new class of cannabinoid CB1 receptor ligands. This groundbreaking research led to the identification of a lead compound and initiated initial structure-activity relationship (SAR) studies. The work was featured in Bioorganic & Medicinal Chemistry Letters (Volume 17, Issue 1, pages 272–7). Further insights into this study can be obtained through doi:10.1016/j.bmcl.2006.09.049, and the PMID is 17027269.
- March 2008 marked significant progress in the field of arylsulfonamides with Ermann M, Riether D, Walker ER, and their team focusing on arylsulfonamide CB2 receptor agonists. This research involved the optimization of CB2 receptor selectivity and was documented in Bioorganic & Medicinal Chemistry Letters (Volume 18, Issue 5, pages 1725–9). For a deeper understanding, the study can be explored via doi:10.1016/j.bmcl.2008.01.042, with the PMID being 18255291.
- In May 2008, Worm K, Zhou QJ, Saeui CT, and their collaborators delved into sulfamoyl benzamides as novel CB2 cannabinoid receptor ligands. This work aimed to uncover their potential and was documented in Bioorganic & Medicinal Chemistry Letters (Volume 18, Issue 9, pages 2830–5). For further details, you can refer to doi:10.1016/j.bmcl.2008.04.006, with the PMID being 18430570.
- In January 2009, Goodman AJ, Ajello CW, Worm K, and their team optimized the amide functionality of CB2 selective sulfamoyl benzamides. Their research was featured in Bioorganic & Medicinal Chemistry Letters (Volume 19, Issue 2, pages 309–13). To explore this aspect in depth, refer to doi:10.1016/j.bmcl.2008.11.091, with the PMID being 19091565.
- January 2010 witnessed significant developments as Sellitto I, Le Bourdonnec B, Worm K, and their colleagues introduced novel sulfamoyl benzamides as selective CB(2) agonists with improved in vitro metabolic stability. This research was documented in Bioorganic & Medicinal Chemistry Letters (Volume 20, Issue 1, pages 387–91). For more comprehensive information, you can access the study through doi:10.1016/j.bmcl.2009.10.062, with the PMID being 19919895.
- In a US application published on Nov 20, 2007, Roland E. Dolle, Karin Worm, and Q. Jean Zhou introduced sulfamoyl benzamide derivatives and methods of their use, providing essential insights into the development of this class of compounds.
- In 2020, Brandt SD, Kavanagh PV, Westphal F, Dreiseitel W, and their research team explored synthetic cannabinoid receptor agonists, presenting analytical profiles and introducing QMPSB, QMMSB, QMPCB, 2F-QMPSB, QMiPSB, and SGT-233. Their research was published in Drug Testing and Analysis (Volume 13, Issue 1, pages 175–196). For further details, consult doi:10.1002/dta.2913, with the PMID being 32880103.