Beautiful Plants For Your Interior
Summary
Phenibut, available under various brand names such as Anvifen, Fenibut, and Noofen, is a central nervous system depressant known for its anxiolytic properties. It is prescribed to address conditions like anxiety, insomnia, and other related indications. Typically administered orally in tablet form, it can also be given intravenously.
Phenibut may induce several side effects, including sedation, drowsiness, nausea, irritability, agitation, dizziness, and occasional euphoria, leading to reduced anxiety. In some cases, it may cause headaches. Overdosing on phenibut can result in pronounced central nervous system depression, potentially leading to unconsciousness.
This medication shares a structural similarity with the neurotransmitter γ-aminobutyric acid (GABA), making it a GABA analog. Phenibut is believed to function as a GABAB receptor agonist, akin to substances like baclofen and γ-hydroxybutyrate (GHB). However, in lower concentrations, phenibut exhibits a mild increase in dopamine levels in the brain, providing stimulating effects alongside its anxiolytic properties.
Initially developed in the Soviet Union, phenibut was introduced for medical use in the 1960s. Presently, it is authorized for medical purposes in countries like Russia, Ukraine, Belarus, Kazakhstan, and Latvia. However, it lacks approval for clinical use in the United States and most of Europe. Despite this, it is available for purchase online as a supplement and is promoted as a nootropic.
Phenibut is occasionally used recreationally and can lead to feelings of euphoria. It is essential to note that it carries the potential for addiction, dependence, and withdrawal symptoms. In Australia, it is classified as a controlled substance, and there are ongoing discussions about reevaluating its legal status in parts of Europe.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1078-21-3 3060-41-1 (hydrochloride) |
|---|---|
| PubChem CID | 14113 |
| ChemSpider | 13491 |
| UNII | T2M58D6LA8 |
| KEGG | D10509 |
| ChEMBL | ChEMBL315818 |
| CompTox Dashboard (EPA) | DTXSID70870838 |
| ECHA InfoCard | 100.012.800 |
| Chemical and physical data | |
| Formula | C10H13NO2 |
| Molar mass | 179.219 g·mol−1 |
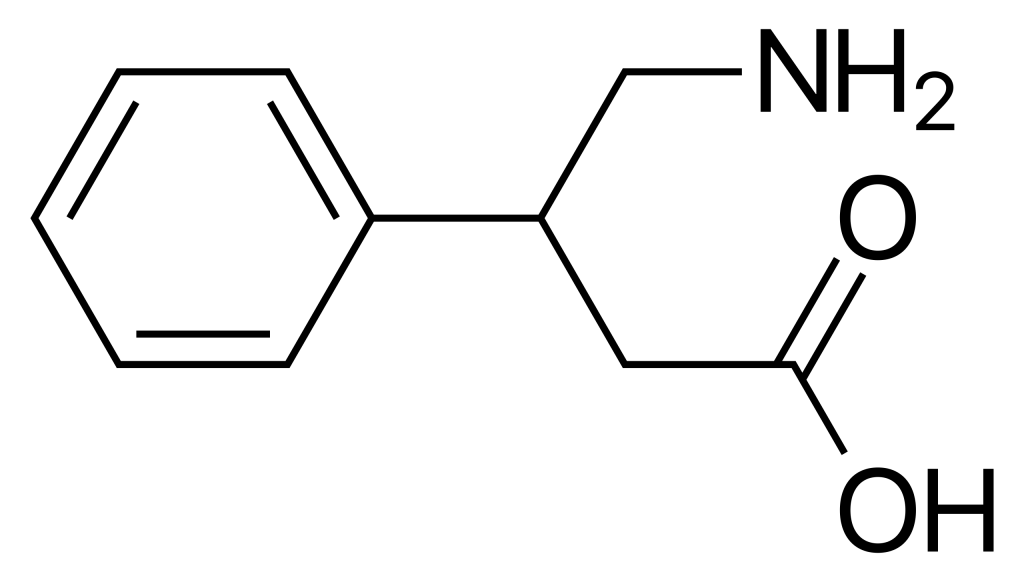
Medical uses
Phenibut serves as a pharmaceutical treatment for anxiety and sleep disorders in Russia, Ukraine, Belarus, and Latvia, including insomnia. Additionally, it finds application in addressing a range of conditions such as asthenia, depression, alcoholism, alcohol withdrawal syndrome, post-traumatic stress disorder, stuttering, tics, vestibular disorders, Ménière’s disease, dizziness, motion sickness prevention, and pre/post-surgical anxiety management.
Available Forms: Phenibut is accessible in the pharmaceutical market as 250 mg or 500 mg oral tablets and as a solution with a concentration of 10 mg/mL for intravenous infusion. Notably, in the United States, dietary supplements labeled to contain phenibut have been scrutinized, revealing variations in phenibut content ranging from none to exceeding 1,100 mg per serving.
Side effects
Phenibut is typically well-tolerated. Potential side effects may encompass sedation, drowsiness, nausea, irritability, agitation, anxiety, dizziness, headache, and allergic reactions like skin rash and itching. In instances of high doses, it might lead to motor incoordination, loss of balance, and hangovers. Due to its CNS depressant properties, individuals using phenibut should abstain from engaging in potentially hazardous activities such as operating heavy machinery. Extended and high-dose use of phenibut may necessitate monitoring of liver and blood function due to the potential risk of fatty liver disease and eosinophilia.
Overdose
In cases of overdose, phenibut can induce severe symptoms such as pronounced drowsiness, nausea, vomiting, eosinophilia, decreased blood pressure, renal impairment, and, at doses exceeding 7 grams, fatty liver degeneration. There are no specific antidotes available for addressing phenibut overdose, and individuals who have overdosed, mainly recreational users, may exhibit lethargy, drowsiness, agitation, delirium, tonic-clonic seizures, reduced consciousness, or even unconsciousness, and unresponsiveness. The management of phenibut overdose typically involves measures like administering activated charcoal, gastric lavage, inducing vomiting, and providing symptom-based treatment. At the same time, there have been three documented cases of associated deaths where phenibut was detected in the users’ system; only one of these cases singularly involved phenibut.
Dependency and Withdrawal
Frequent use of phenibut can lead to the development of tolerance and subsequent dependence. Discontinuation of phenibut use may result in withdrawal symptoms, which can be particularly severe in recreational users who have been taking high doses. These withdrawal symptoms may encompass intense rebound anxiety, insomnia, anger, irritability, agitation, visual and auditory hallucinations, and, in extreme cases, acute psychosis. Baclofen has shown efficacy in the treatment of phenibut dependence.
Interactions
Phenibut has the potential to enhance and prolong the effects of other central nervous system depressants, including anxiolytics, antipsychotics, sedatives, opioids, anticonvulsants, and alcohol.
Pharmacology
Pharmacodynamics
In the realm of pharmacodynamics, phenibut exhibits its activity at biological targets, particularly at GABA (Gamma-Aminobutyric Acid) and related receptors. Here are the relative inhibitory concentration (IC50) values of various compounds in rat brains:
- GABA: GABAB: 0.08, GABAA: 0.12
- GHB (Gamma-Hydroxybutyric Acid): GABAB: >100, GABAA: >100
- GABOB (Gamma-Amino-β-Hydroxybutyric Acid): GABAB: 1.10, GABAA: 1.38
- Phenibut: GABAB: 9.6, GABAA: >100
- 4-F-phenibut: GABAB: 1.70, GABAA: >100
- Baclofen: GABAB: 0.13, GABAA: >100
- (R)-Baclofen: GABAB: 0.13, GABAA: >100
- (S)-Baclofen: GABAB: 74.0, GABAA: >100
Phenibut acts as a full agonist at the GABAB receptor, much like baclofen. However, it possesses a substantially lower affinity for the GABAB receptor compared to baclofen, which results in the utilization of significantly higher doses. Additionally, it’s noteworthy that (R)-Phenibut exhibits over a 100-fold higher affinity for the GABAB receptor than (S)-Phenibut, making (R)-Phenibut the active enantiomer at the GABAB receptor.
In terms of binding to α2δ subunit-containing VDCCs (Voltage-Dependent Calcium Channels), halibut and its enantiomers interact with similar affinity. Furthermore, this interaction classifies phenibut as a gabapentinoid, similar to gabapentin and pregabalin, due to its capability to bind and block α2δ subunit-containing VDCCs.
Pharmacokinetics
Currently, there is limited information available on the clinical pharmacokinetics of phenibut. It is reported to be well-absorbed, and it demonstrates wide distribution throughout the body, effectively crossing the blood-brain barrier. Approximately 0.1% of an administered dose of phenibut is believed to penetrate the brain, with a greater extent of penetration noted in young individuals and the elderly. After a single 250 mg dose in healthy volunteers, the drug exhibits an elimination half-life of roughly 5.3 hours, with about 63% of the drug being excreted unchanged in the urine.
There is some information about the pharmacokinetics of phenibut in recreational users who take higher doses, such as 1-3 grams. For these users, the onset of action takes about 2 to 4 hours when administered orally and 20 to 30 minutes when administered rectally. Peak effects are observed 4 to 6 hours following oral ingestion, and the total duration for the oral route is reported to be 15 to 24 hours.
Chemistry
Phenibut is a synthetic aromatic amino acid. This chiral molecule has two potential configurations as (R)- and (S)-enantiomers. Its structure and analogs are closely related to GABA and other GABA analogs, such as baclofen, 4-fluorophenibut, halibut, pregabalin, gabapentin, and GABOB.
History
Phenibut was initially synthesized at the A. I. Herzen Leningrad Pedagogical Institute (USSR) by Professor Vsevolod Perekalin’s research team. It was subsequently tested at the Institute of Experimental Medicine, USSR Academy of Medical Sciences, and introduced into clinical use in Russia during the 1960s.
Society and Culture
Phenibut goes by alternate spellings such as fenibut and phenybut. Although it hasn’t been assigned an International Nonproprietary Name (INN), it is marketed under different brand names in Russia, Ukraine, Belarus, and Latvia, including Anvifen, Fenibut, Bifren, and Noofen. Phenibut is approved for medical use in these countries but is not authorized as a medication in the European Union, the United States, or Australia. In regions where it’s not a licensed pharmaceutical, phenibut is often sold online without a prescription as a “nutritional supplement.”
Recreational Use
Phenibut is employed recreationally for its potential to induce euphoria, anxiolysis, and enhanced sociability. It is favored for remaining undetected in routine urinalysis tests. Due to its delayed onset of action, first-time users sometimes mistakenly take additional doses, believing the initial dose could have been more effective. It is typically ingested orally, although there are rare reports of rectal administration and a painful insufflation case causing swollen nostrils.
Legal Status
As of 2021, phenibut is classified as a controlled substance in Australia, France, Hungary, Italy, and Lithuania. Its legal status in Europe has been proposed for reconsideration due to its recreational potential. In February 2018, the Australian Therapeutic Goods Administration prohibited phenibut, categorizing it as a Schedule 9 substance due to health concerns related to withdrawal and overdose.
On November 14, 2018, Hungary added phenibut and 10 other substances to its New Psychoactive Substances ban list. Italy followed suit on August 26, 2020, adding phenibut to its New Psychoactive Substances ban list. France prohibited phenibut on September 18, 2020, listing it as a controlled psychoactive substance, thus disallowing production, sale, storage, and use.
In the United States, phenibut is not classified as a controlled substance. However, dietary supplements containing phenibut are illegal to introduce into interstate commerce because phenibut is considered a “New Drug.” Any food, supplement, cosmetic, or drug containing phenibut is therefore misbranded. Alabama also scheduled phenibut and tianeptine in 2021 through actions of the Alabama Department of Public Health and the state legislature.
FAQ
1. What is Phenibut?
- Phenibut is a synthetic compound created from the neurotransmitter GABA (Gamma-Aminobutyric Acid). It is known for its potential calming and anxiolytic effects.
2. How is Phenibut Used?
- Phenibut is commonly used as a pharmaceutical drug to manage anxiety, improve sleep, and treat conditions such as insomnia. It’s also used for other indications like asthenia, depression, and vestibular disorders.
3. Where is Phenibut Available?
- Phenibut is approved and available as a medication in Russia, Ukraine, Belarus, and Latvia. In other countries, it is sold online as a nutritional supplement without a prescription.
4. What Are the Available Forms of Phenibut?
- Phenibut is available in the form of oral tablets, usually in 250 mg or 500 mg doses, as well as a solution for infusion at a concentration of 10 mg/mL.
5. What Are the Possible Side Effects of Phenibut?
- Phenibut is generally well-tolerated, but potential side effects may include sedation, nausea, irritability, agitation, dizziness, and headaches. At high doses, it can lead to motor incoordination and hangovers.
6. Is Phenibut Safe to Use with Alcohol?
- No, combining Phenibut with alcohol is not recommended as it can lead to undesirable side effects and potentially dangerous interactions.
7. Can You Overdose on Phenibut?
- Yes, an overdose of Phenibut can lead to symptoms such as severe drowsiness, nausea, vomiting, and, in severe cases, fatty liver degeneration. There are no specific antidotes for Phenibut overdose.
8. Does Phenibut Lead to Dependency and Withdrawal?
- Yes, tolerance can develop with repeated use, potentially leading to dependency. When discontinuing Phenibut, withdrawal symptoms may occur, including severe rebound anxiety, insomnia, irritability, and, in some cases, hallucinations and acute psychosis.
9. What Are the Interactions of Phenibut?
- Phenibut can interact with other central nervous system depressants, such as anxiolytics, antipsychotics, sedatives, opioids, and alcohol, which can lead to enhanced effects and potential risks.
10. Is Phenibut Legal Everywhere?
– No, the legal status of Phenibut varies by country. While it is not a controlled substance in the United States, it is regulated in several European countries, including France, Hungary, Italy, and Lithuania.
11. What Is the History of Phenibut?
– Phenibut was initially synthesized in the USSR in the 1960s and was introduced into clinical use. It has a history of medicinal use for anxiety and sleep-related disorders.
12. Can Phenibut Be Used Recreationally?
– Yes, Phenibut is sometimes used recreationally due to its potential to induce euphoria, anxiolysis, and increased sociability. It’s also attractive because it often goes undetected in routine drug tests.
13. Is Phenibut Suitable for Self-Medication?
– While it is available as a nutritional supplement in some regions, self-medication should be approached with caution. It’s advisable to consult with a healthcare professional before using Phenibut for medical purposes.
14. Are There Specific Brand Names for Phenibut?
– Yes, Phenibut is marketed under different brand names in Russia, Ukraine, Belarus, and Latvia, including Anvifen, Fenibut, Bifren, and Noofen.
References
- Drobizhev MY, Fedotova AV, Kikta SV, Antohin EY (2016). “Phenomenon of aminophenylbutyric acid”. Russian Medical Journal (in Russian). 2017 (24): 1657–1663. ISSN 1382-4368.
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures, and Bibliographies. Springer. pp. 69–. ISBN 978-1-4757-2085-3.
- Owen DR, Wood DM, Archer JR, Dargan PI (September 2016). “Phenibut (4-amino-3-phenyl-butyric acid): Availability, prevalence of use, desired effects, and acute toxicity”. Drug and Alcohol Review. 35 (5): 591–6. doi:10.1111/dar.12356. PMID 26693960.
- Nutrition, Center for Food Safety and Applied (6 March 2023). “Phenibut in Dietary Supplements”. FDA.
- Ozon Pharm, Fenibut (PDF), archived from the original (PDF) on 16 September 2017, retrieved 15 September 2017.
- Lapin I (2001). “Phenibut (beta-phenyl-GABA): a tranquilizer and nootropic drug”. CNS Drug Reviews. 7 (4): 471–81. doi:10.1111/j.1527-3458.2001.tb00211.x. PMC 6494145. PMID 11830761.
- Регистр лекарственных средств России ([Russian Medicines Register]). “Фенибут (Phenybutum)” [Fenibut (Phenybutum)] (in Russian). Retrieved 15 September 2017.
- Lapin I (7 June 2006). “Phenibut (beta-phenyl-GABA): a tranquilizer and nootropic drug”. CNS Drug Reviews. 7 (4): 471–81. doi:10.1111/j.1527-3458.2001.tb00211.x. PMC 6494145. PMID 11830761.
- Cohen PA, Ellison RR, Travis JC, Gaufberg SV, Gerona R (April 2022). “Quantity of phenibut in dietary supplements before and after FDA warnings”. Clinical Toxicology. 60 (4): 486–488. doi:10.1080/15563650.2021.1973020. PMID 34550038.
- Sivchik VV, Grygoryan HO, Survilo VL, Trukhachova TV (2012), Синтез γ-амино-β-фенилмасляной кислоты (фенибута) [Synthesis of β-phenyl-γ-aminobutyric acid (phenibut)] (PDF) (in Russian).
- Graves JM, Dilley J, Kubsad S, Liebelt E (September 2020). “Notes from the Field: Phenibut Exposures Reported to Poison Centers – United States, 2009–2019”. MMWR. Morbidity and Mortality Weekly Report. 69 (35): 1227–1228. doi:10.15585/mmwr.mm6935a5. PMC 7470459. PMID 32881852.
- Samokhvalov AV, Paton-Gay CL, Balchand K, Rehm J (February 2013). “Phenibut dependence”. BMJ Case Reports. 2013: bcr2012008381. doi:10.1136/bcr-2012-008381. PMC 3604470. PMID 23391959.
- Bowery NG, Hill DR, Hudson AL (January 1983). “Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes”. British Journal of Pharmacology. 78 (1): 191–206. doi:10.1111/j.1476-5381.1983.tb09380.x. PMC 2044790. PMID 6297646.
- GABAb Receptor Pharmacology: A Tribute to Norman Bowery: A Tribute to Norman Bowery. Academic Press. 21 September 2010. pp. 25–. ISBN 978-0-12-378648-7.
- Dambrova M, Zvejniece L, Liepinsh E, Cirule H, Zharkova O, Veinberg G, Kalvinsh I (March 2008). “Comparative pharmacological activity of optical isomers of phenibut”. European Journal of Pharmacology. 583 (1): 128–134. doi:10.1016/j.ejphar.2008.01.015. PMID 18275958.
- Allan RD, Bates MC, Drew CA, Duke RK, Hambley TW, Johnston GA, et al. (1990). “A new synthesis resolution and in vitro activities of (R)- and (S)-β-Phenyl-Gaba”. Tetrahedron. 46 (7): 2511–2524. doi:10.1016/S0040-4020(01)82032-9. ISSN 0040-4020.
- Zvejniece L, Vavers E, Svalbe B, Veinberg G, Rizhanova K, Liepins V, et al. (October 2015). “R-phenibut binds to the α2-δ subunit of voltage-dependent calcium channels and exerts gabapentin-like anti-nociceptive effects”. Pharmacology, Biochemistry, and Behavior. 137: 23–9. doi:10.1016/j.pbb.2015.07.014. PMID 26234470.
- Vavers E, Zvejniece L, Svalbe B, Volska K, Makarova E, Liepinsh E, et al. (November 2016). “The neuroprotective effects of R-phenibut after focal cerebral ischemia”. Pharmacological Research. 113 (Pt B): 796–801. doi:10.1016/j.phrs.2015.11.013. PMID 26621244.
- Schifano F, Orsolini L, Duccio Papanti G, Corkery JM (February 2015). “Novel psychoactive substances of interest for psychiatry”. World Psychiatry. 14 (1): 15–26. doi:10.1002/wps.20174. PMC 4329884. PMID 25655145.
- Perfilova VN, Popova TA, Prokofiev II, Mokrousov IS, Ostrovskii OV, Tyurenkov IN (June 2017). “Effect of Phenibut and Glufimet, a Novel Glutamic Acid Derivative, on Respiration of Heart and Brain Mitochondria from Animals Exposed to Stress against the Background of Inducible NO-Synthase Blockade”. Bulletin of Experimental Biology and Medicine. 163 (2): 226–229. doi:10.1007/s10517-017-3772-4. PMID 28726197.
- Khaunina RA, Lapin IP (1976). “Fenibut, a new tranquilizer”. Pharmaceutical Chemistry Journal. 10 (12): 1703–1705. doi:10.1007/BF00760021. ISSN 0091-150X.