Summary
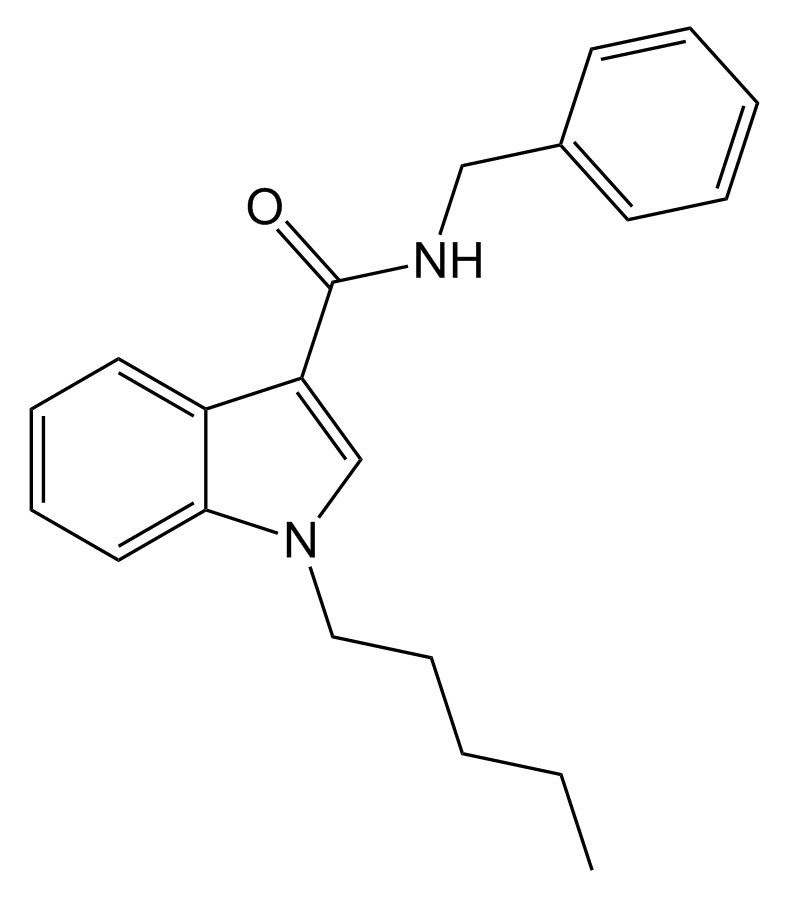
SDB-006 is a pharmacological agent known for its potent agonistic activity on cannabinoid receptors. Its effectiveness is noteworthy, with an EC50 of 19 nM for human CB2 receptors and 134 nM for human CB1 receptors. This compound was initially uncovered while investigating its analogue, SDB-001, which had gained notoriety as “2NE1” when sold illicitly. The metabolic pathways of SDB-006 have been comprehensively documented in the scientific literature.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 695213-59-3 |
|---|---|
| PubChem CID | 2227769 |
| ChemSpider | 1665835 |
| UNII | MM02A5A302 |
| CompTox Dashboard (EPA) | DTXSID501009997 |
| Chemical and physical data | |
| Formula | C21H24N2O |
| Molar mass | 320.436 g·mol−1 |
FAQ
1. What is SDB-006?
- SDB-006 is a pharmacological compound known for its potent agonistic effects on cannabinoid receptors, specifically the CB1 and CB2 receptors.
2. What are cannabinoid receptors (CB1 and CB2)?
- Cannabinoid receptors CB1 and CB2 are part of the endocannabinoid system in the human body. They are significant in various physiological processes, including pain modulation, immune function, and mood regulation.
3. What is the EC50 of SDB-006 for human CB1 and CB2 receptors?
- SDB-006 has an EC50 (effective concentration for 50% of the maximum response) of 19 nM for human CB2 receptors and 134 nM for human CB1 receptors. This indicates its potency in activating these receptors.
4. How was SDB-006 discovered?
- SDB-006 came to light during research into a related compound, SDB-001, which was being sold illicitly under the street name “2NE1.” The investigation led to the identification of SDB-006.
5. Is SDB-006 safe for use or consumption?
- The safety of SDB-006 for human consumption is not well-established. Synthetic cannabinoids like SDB-006 can have unpredictable effects and potential health risks. Using such compounds should be approached with caution.
6. What is known about the metabolism of SDB-006?
- The metabolism of SDB-006 has been studied and documented in scientific literature. This research provides insights into how the compound is processed in the body, which is essential for understanding its effects and potential risks.
7. Is SDB-006 legal to possess or use?
- The legal status of SDB-006 varies by country and jurisdiction. It is important to check local laws and regulations regarding its possession and use, as it may be considered a controlled or illicit substance in some areas.
8. Where can I find more information about SDB-006?
- To learn more about SDB-006, its effects, and its legal status, refer to reputable sources, including scientific literature, government health agencies, and substance abuse resources. Always rely on accurate and up-to-date information.
References
- Research on Bioisosteric Fluorine in Synthetic Cannabinoids – In August 2015, Banister SD and his team investigated the effects of bioisosteric fluorine in a range of synthetic cannabinoid designer drugs. This comprehensive study included compounds like JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135. Their findings, published in ACS Chemical Neuroscience (Volume 6, Issue 8), shed light on the impact of fluorine substitutions in these substances. [doi:10.1021/acschemneuro.5b00107] [PMID 25921407].
- Chemistry and Pharmacology of Synthetic Cannabinoid SDB-006 – In January 2018, Banister SD and colleagues delved into the chemistry and pharmacology of SDB-006, as well as its regioisomeric fluorinated and methoxylated analogs. This research, featured in Drug Testing and Analysis (Volume 10, Issue 7), provides insights into the structure-activity relationships of these synthetic cannabinoids. [doi:10.1002/dta.2362] [PMID 29350472].
- Synthesis and Pharmacological Evaluation of Adamantane-Derived Indoles – In July 2013, Banister SD and collaborators focused on the synthesis and pharmacological evaluation of adamantane-derived indoles, specifically as cannabimimetic drugs of abuse. This study, published in ACS Chemical Neuroscience (Volume 4, Issue 7), offers valuable information on the pharmacological properties of these compounds. [doi:10.1021/cn400035r] [PMCID 3715837] [PMID 23551277].
- In Vitro Metabolism of Synthetic Cannabinoid SDB-006 – In July 2017, Diao X, Carlier J, and Scheidweiler KB explored the in vitro metabolism of the synthetic cannabinoid SDB-006 in human hepatocytes using high-resolution mass spectrometry. Their research, documented in Forensic Toxicology (Volume 35, Issue 2), provides insights into the metabolic pathways of this compound. [doi:10.1007/s11419-016-0350-9] [S2CID 26558377].