Summary
Thiopropamine is a stimulant substance that is an analogue of amphetamine, featuring replacing the phenyl ring with thiophene. While it shares stimulant properties with amphetamine, its potency is approximately one-third less. Additionally, variations such as the N-methyl and thiophene-3-yl analogues are known to be somewhat more potent but generally remain less powerful than their amphetamine counterparts.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 30433-93-3 |
|---|---|
| PubChem CID | 6484133 |
| ChemSpider | 4984575 |
| UNII | N60H4ZDD14 |
| ChEMBL | ChEMBL95500 |
| CompTox Dashboard (EPA) | DTXSID80424381 |
| Chemical and physical data | |
| Formula | C7H11NS |
| Molar mass | 141.23 g·mol−1 |
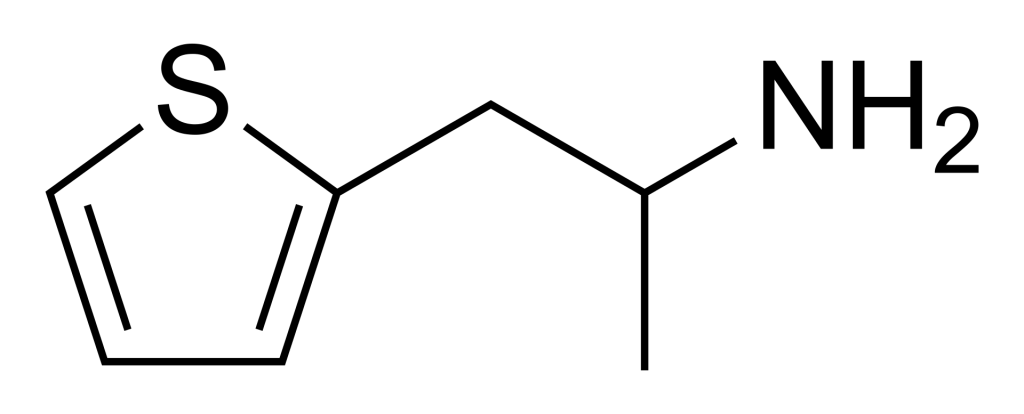
Pharmacology
Much like amphetamine and its various analogues, it is pretty probable that thiopropamine functions as a norepinephrine-dopamine reuptake inhibitor and a releasing agent. However, it’s important to note that further citation and research are needed to confirm this.
The metabolism of thiopropamine likely involves the conversion into active compounds, notably 4-hydroxymethiopropamine and thiophene S-oxides. These compounds are subject to deamination by CYP2C in the liver, transforming them into inactive 1-(Thiophen-2-yl)-2-propan-2-one, a derivative of phenylacetone. It’s worth mentioning that propane-2-amines, like thiopropamine, generally evade metabolism by monoamine oxidases and, in most cases, can even act as competitive monoamine oxidase inhibitors.
FAQ
1. What is Thiopropamine?
- Thiopropamine is a substance categorized as a stimulant. It serves as an analogue of amphetamine, featuring a structural modification where the phenyl ring is replaced by thiophene.
2. What are the effects of Thiopropamine?
- Thiopropamine shares stimulant properties with amphetamine, although it is generally around one-third less potent. It is likely to have norepinephrine-dopamine reuptake inhibiting and releasing effects, similar to amphetamine and its analogues.
3. How does Thiopropamine metabolize in the body?
- Thiopropamine is likely metabolized into active compounds, including 4-hydroxymethiopropamine and thiophene S-oxides. These metabolites are further transformed into inactive 1-(Thiophen-2-yl)-2-propan-2-one by the enzyme CYP2C in the liver. This compound is a derivative of phenylacetone.
4. Are there any potential interactions or concerns with Thiopropamine use?
- As with any substance, Thiopropamine may have potential interactions with other drugs or substances. Additionally, its use may be associated with various health risks and side effects. It is essential to exercise caution and seek professional advice if considering its use.
5. Is Thiopropamine widely available or legal to use?
- The availability and legal status of Thiopropamine can vary by region and country. It is crucial to check your area’s specific laws and regulations regarding the possession, sale, or use of this substance.
6. Is there ongoing research or scientific interest in Thiopropamine?
- Information about Thiopropamine may be limited, and research in this area may not be as extensive as other substances. It’s advisable to consult scientific literature and research publications for the latest findings and updates.
7. What are the potential risks associated with Thiopropamine use?
- The use of Thiopropamine may carry risks such as adverse health effects, addiction potential, and potential interactions with other substances. It’s essential to be well-informed and exercise caution.
8. Where can I find more information about Thiopropamine?
- To obtain comprehensive and up-to-date information about Thiopropamine, consider consulting scientific literature, medical resources, and government agencies responsible for regulating substances. Additionally, seeking guidance from healthcare professionals is advisable for specific concerns or questions.
References
- In July 1941, Alles GA and Feigen GA conducted research on the “Comparative Physiological Actions of Phenyl-, Thienyl- and Furylisopropylamines.” Their findings were published in the “Journal of Pharmacology and Experimental Therapeutics,” Volume 72 (3), pages 265–75.
- Campaigne E and McCarthy WC, in September 1954, explored “3-Substituted Thiophenes. VIII. 3-Thienylalkylamines.” This research was featured in the “Journal of the American Chemical Society,” Volume 76 (17), pages 4466–4468, with a DOI of 10.1021/ja01646a054.
- In February 1997, Treiber A, Dansette PM, El Amri H, Girault JP, Ginderow D, Mornon JP, and Mansuy D delved into the “Chemical and Biological Oxidation of Thiophene.” Their comprehensive work led to the complete characterization of “Thiophene S-Oxide Dimers” and provided evidence for the role of “Thiophene S-Oxide as an Intermediate in Thiophene Metabolism in Vivo and in Vitro.” This research was published in the “Journal of the American Chemical Society,” Volume 119 (7), pages 1565–1571, with a DOI of 10.1021/ja962466g.
- Dansette PM, Thang DC, el Amri H, and Mansuy D, in August 1992, contributed to the understanding of thiophene metabolism with their research on “Evidence for thiophene-S-oxide as a primary reactive metabolite of thiophene in vivo.” They explored the formation of “dihydrothiophene sulfoxide mercapturic acid” and published their findings in “Biochemical and Biophysical Research Communications,” Volume 186 (3), pages 1624–30, with a DOI of 10.1016/S0006-291X(05)81594-3 and a PMID of 1510686.
- Yamada H, Shiiyama S, Soejima-Ohkuma T, Honda S, Kumagai Y, Cho AK, et al., in February 1997, conducted research on the “Deamination of amphetamines by cytochromes P450.” They explored substrate specificity and regioselectivity with microsomes and purified CYP2C subfamily isozymes, which was published in “The Journal of Toxicological Sciences,” Volume 22 (1), pages 65–73, with a DOI of 10.2131/jts.22.65 and a PMID of 9076658.
- In September 1976, Mantle TJ, Tipton KF, and Garrett NJ conducted research on the “Inhibition of monoamine oxidase by amphetamine and related compounds,” which was published in “Biochemical Pharmacology,” Volume 25 (18), pages 2073–7, with a DOI of 10.1016/0006-2952(76)90432-9 and a PMID of 985546.
- Miller HH, Shore PA, and Clarke DE, in May 1980, investigated “In vivo monoamine oxidase inhibition by d-amphetamine.” Their research was published in “Biochemical Pharmacology,” Volume 29 (10), pages 1347–54, with a DOI of 10.1016/0006-2952(80)90429-3 and a PMID of 6901611.