Summary
U-47700 also recognized as U4, pink heroin, pinky, or pink, is an opioid analgesic drug initially developed by a team at Upjohn during the 1970s. In animal models, it boasts approximately 7.5 times the potency of morphine.
A physical sample of U-47700 is available for reference.
This compound is a structural isomer of the earlier opioid AH-7921 and is the culmination of extensive research into the quantitative structure-activity relationship of its chemical framework. Upjohn systematically explored the crucial structural components that contributed to its potent activity and secured more than a dozen related patents, each aimed at enhancing specific structural elements. Eventually, they identified U-47700 as the most active variant among them.
U-47700 has served as the foundational compound for the development of selective kappa-opioid receptor ligands, such as U-50488, U-51754 (featuring a pyrrolidine substituent instead of dimethylamine), and U-69,593, which share remarkably similar structural characteristics. While these selective kappa ligands are not employed for medical purposes, they play a significant role in scientific research.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 82657-23-6 |
|---|---|
| PubChem CID | 13544016 |
| ChemSpider | 23113403 |
| UNII | 6IY4WX208T |
| ChEMBL | ChEMBL277572 |
| CompTox Dashboard (EPA) | DTXSID001014181 |
| Chemical and physical data | |
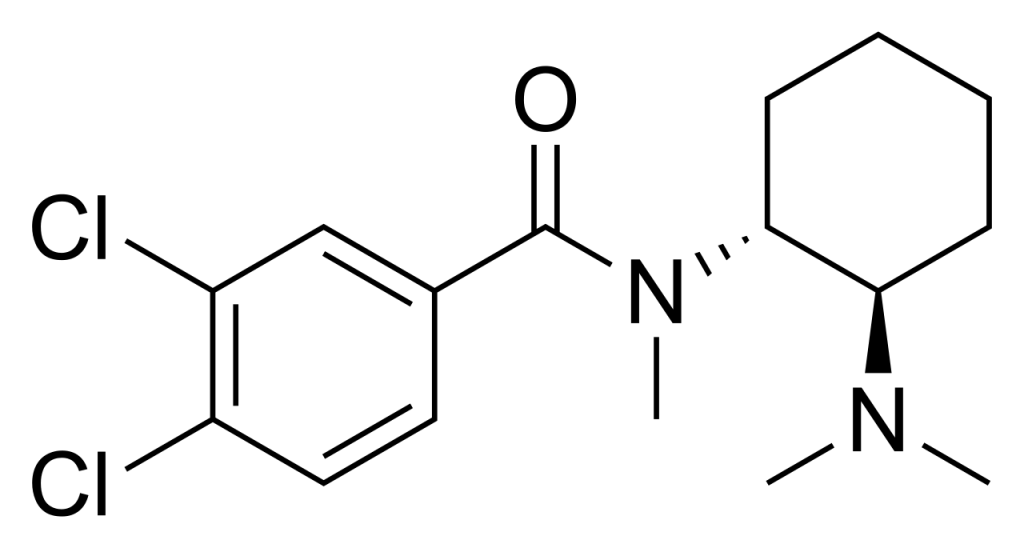
| Formula | C16H22Cl2N2O |
| Molar mass | 329.27 g·mol−1 |
Pharmacology
U-47700 acts as an agonist for the μ-opioid receptor (Ki 11.1 ± 0.4nM), displaying notably reduced affinity for the κ-opioid receptor (Ki 287 ± 24nM) and δ-opioid receptor (Ki 1220 ± 82nM). In rats, U-47700 exhibits approximately 10-fold greater potency than morphine, although its binding affinity at all three opioid receptors is 2-4 times weaker compared to morphine.
The human metabolism of U-47700 entails mono- anddidesmethylation followed by hydroxylation. Importantly, the desmethyl metabolites of U-47700 exhibit minimal affinity for opioid receptors and are not believed to contribute significantly to U-47700’s pharmacological activity.
Side effects
While U-47700 has not been subjected to human studies, it is anticipated to elicit effects akin to those observed with potent opioid agonists. These effects encompass robust pain relief, sedation, feelings of euphoria, constipation, itching, and the potential for harmful or fatal respiratory depression. Additionally, the use of U-47700 has been linked to instances of tachycardia. The development of tolerance and dependence is also expected.
Incidents of Deaths:
Tragically, fatalities have been associated with U-47700 use. In Belgium and Germany, one fatality each resulted from the combined consumption of U-47700 with fentanyl and flubromazepam, respectively.[34][35][36] Ireland reported one death, while another occurred in Italy.[37][38] In the United States, U-47700 was initially linked to 17 opioid overdoses and several deaths in April 2016, with at least 15 confirmed fatalities as of September 2016. By December 2017, the number of fatalities associated with U-47700 had surged to at least 46 cases. Notably, U-47700 was detected alongside fentanyl during the autopsy of the renowned American artist Prince in 2016.
Detection in Biological Fluids:
U-47700 may be quantified in serum, plasma, blood, or urine to monitor its abuse potential, validate poisoning diagnoses, or aid in medicolegal death investigations. In individuals who have consumed the substance, serum or blood U-47700 concentrations typically fall within the range of 10–250 μg/L, while in cases of acute overdose leading to death, these concentrations can range from 100–1,500 μg/L. The detection process generally involves analysis employing liquid chromatography-mass spectrometry.
Legal status
After being sold as a designer drug, Sweden declared U-47700 illegal on January 26, 2016.
In Ohio, Governor John Kasich issued an executive order on May 3, 2016, to emergency schedule U-47700.
Florida Attorney General Pam Bondi issued an emergency rule on September 27, 2016, to emergency schedule U-47700 in Florida due to concerns about public health and safety.
In response to perceived threats to public health and safety, the U.S. Drug Enforcement Administration (DEA) classified U-47700 as a Schedule I controlled substance, effective from November 14, 2016. Subsequently, in April 2018, U-47700 was permanently placed in Schedule I.
South Dakota included U-47700 in Schedule I of its Controlled Substance Schedule, with Governor Daugaard signing it into law on February 9, 2017.
FAQ
1. What is U-47700?
U-47700, also known by various street names like U4, pink heroin, pinky, and pink, is a synthetic opioid analgesic. It was initially developed in the 1970s by Upjohn, a pharmaceutical company.
2. What are the effects of U-47700?
U-47700 produces effects similar to other potent opioids, including pain relief, sedation, euphoria, constipation, itching, and, most critically, respiratory depression, which can be life-threatening.
3. Is U-47700 legal?
U-47700 is classified as a controlled substance in several countries and regions. Laws regarding its legality can vary, but in many places, it is illegal to manufacture, possess, or distribute U-47700.
4. Is U-47700 safe to use?
No, U-47700 is not safe for use. It is associated with a high risk of respiratory depression, overdose, and death, even at relatively low doses. Using U-47700 is extremely dangerous and not recommended.
5. What is the history of U-47700’s legal status?
The legal status of U-47700 has changed over the years. It was initially sold as a designer drug but has since been classified as a controlled substance in many places. Laws regarding its status can change, so it’s essential to check local regulations for the most up-to-date information.
6. Is U-47700 addictive?
Yes, like other opioids, U-47700 has a high potential for tolerance, dependence, and addiction. Using it regularly can lead to physical and psychological dependence.
7. Are there any reported deaths associated with U-47700 use?
Yes, there have been numerous reported deaths associated with U-47700 use. It has been linked to overdoses and fatalities, often when used in combination with other substances.
8. How can U-47700 be detected in the body?
U-47700 can be detected in biological fluids like blood, serum, plasma, or urine using specialized testing methods, such as liquid chromatography-mass spectrometry. Concentrations can vary depending on the individual’s use and circumstances.
9. Is there any legitimate medical use for U-47700?
U-47700 is not approved for medical use and is not prescribed by healthcare professionals. It is considered a dangerous substance with no recognized therapeutic benefits.
10. What should I do if I suspect someone has used U-47700?
If you suspect someone has used U-47700 and is experiencing symptoms of overdose, such as difficulty breathing, extreme drowsiness, or loss of consciousness, seek immediate medical attention. Do not attempt to treat an overdose on your own.
11. Where can I get more information about U-47700?
For more information about U-47700, its legal status in your area, and its risks, consult your local health department, law enforcement agencies, or substance abuse treatment centers. Additionally, medical professionals and addiction counselors can provide valuable guidance and resources.
References
1. Szmuszkovicz J (4 July 1978). “Patent US4098904 – Analgesic N-(2-aminocycloaliphatic)benzamides”.
2. Cheney BV, Szmuszkovicz J, Lahti RA, Zichi DA (December 1985). “Factors affecting binding of trans-N-[2-(methylamino)cyclohexyl]benzamides at the primary morphine receptor”. Journal of Medicinal Chemistry. 28 (12): 1853–1864. doi:10.1021/jm00150a017. PMID 2999404.
3. Harper NJ, Veitch GB, Wibberley DG (November 1974). “1-(3,4-Dichlorobenzamidomethyl)cyclohexyldimethylamine and related compounds as potential analgesics”. Journal of Medicinal Chemistry. 17 (11): 1188–1193. doi:10.1021/jm00257a012. PMID 4416926.
4. Szmuszkovicz J, Von Voigtlander PF (October 1982). “Benzeneacetamide amines: structurally novel non-m mu opioids”. Journal of Medicinal Chemistry. 25 (10): 1125–1126. doi:10.1021/jm00352a005. PMID 6128415.
5. Alzghari SK, Fleming SW, Rambaran KA, Long JE, Burkhart S, An J, Furmaga J (October 2017). “U-47700: An Emerging Threat”. Cureus. 9 (10): e1791. doi:10.7759/cureus.1791. PMC 5741271. PMID 29282436.
6. Brittain RT, Kellett DN, Neat ML, Stables R (September 1973). “Proceedings: Anti-nociceptive effects in N-substituted cyclohexylmethylbenzamides”. British Journal of Pharmacology. 49 (1): 158P–159P. doi:10.1111/j.1476-5381.1973.tb08279.x. PMC 1776456. PMID 4207044.
7. Michalson ET, Szmuszkovicz J (1989). “Medicinal agents incorporating the 1,2-diamine functionality”. Progress in Drug Research. pp. 135–149. doi:10.1007/978-3-0348-9146-2_6. ISBN 9783034891462. PMID 2687936. {{cite book}}: |journal= ignored (help)
8. Mullins DD (28 June 1966). “Patent US US3258489 – N-(1-aminocyclohexylmethyl)anilines and N-(1-nitrocyclohexylmethyl)anilines”.
9. Harper NJ, Veitch GB (17 August 1976). “Patent US3975443 – 1-(3,4-dichlorobenzamidomethyl)-cyclohexyldimethylamine”.
10. Szmuszkovicz J (3 March 1970). “Patent US3499033 – Ethers of α-phenyl-2-aminocycloalkanemethanols”.
11. Szmuszkovicz J (5 May 1970). “Patent US3510492 – 2-anilino and 2-anilinomethyl cycloalkylamines”.
12. Rynbrandt RH, Skaletzky LL (7 March 1972). “Patent US3647804 – Cycloalkanecarboxamides”.
13. Roll W (23 July 1974). “Patent US3825595 – N-cyclopentyl-N-2-hydroxyalkyl-ring-substituted benzamides”.
14. Harper NJ, Veitch GB (20 September 1977). “Patent US4049663 – Ethylene diamine derivatives”.
15. Collins RJ, Kaplan LJ, Ludens JH, Von Voigtlander PF (9 April 1982). “Patent US4463013 – Oxygen substituted amino-cyclohexyl-benzeneacetamides and -benzamides as water diuretic drugs”.
16. Casy AF, Parfitt RT (1986). “Miscellaneous Groups of Analgesics”. Opioid Analgesics. Springer US. pp. 385–403. doi:10.1007/978-1-4899-0585-7_11. ISBN 9781489905857.
17. Szmuszkovicz J, Zhao S, Totleben MJ, Mizsak SA, Freeman JP (2000). “Phenanthridone Analogs of the Opiate Agonist U-47,700 in the trans-1,2-Diaminocyclohexane Benzamide Series”. Heterocycles. 52 (1): 325–332. doi:10.3987/com-99-s27.
18. Loew G, Lawson J, Toll L, Frenking G, Berzetei-Gurske I, Polgar W (1988). “Structure activity studies of two classes of beta-amino-amides: the search for kappa-selective opioids” (PDF). NIDA Research Monograph. 90: 144–151. PMID 2855852.
19. Szmuszkovicz J (1999). “U-50,488 and the к receptor: A personalized account covering the period 1973 to 1990”. Progress in Drug Research. Vol. 52. Birkhäuser Basel. pp. 167–195. doi:10.1007/978-3-0348-8730-4_4. ISBN 9783034887304. PMID 10396128.
20. Tsibulnikov SY, Maslov LN, Mukhomedzyanov AV, Krylatov AV, Tsibulnikova MR, Lishmanov YB (October 2015). “Prospects of Using of κ-Opioid Receptor Agonists U-50,488 and ICI 199,441 for Improving Heart Resistance to Ischemia/Reperfusion”. Bulletin of Experimental Biology and Medicine. 159 (6): 718–721. doi:10.1007/s10517-015-3057-8. PMID 26519268. S2CID 1046853.
21. Truver MT, Smith CR, Garibay N, Kopajtic TA, Swortwood MJ, Baumann MH (October 2020). “Pharmacodynamics and pharmacokinetics of the novel synthetic opioid, U-47700, in male rats”. Neuropharmacology. 177: 108195. doi:10.1016/j.neuropharm.2020.108195. PMC 7554234. PMID 32533977. S2CID 219559700.
22. Krotulski AJ, Mohr AL, Papsun DM, Logan BK (January 2018). “Metabolism of novel opioid agonists U-47700 and U-49900 using human liver microsomes with confirmation in authentic urine specimens from drug users”. Drug Testing and Analysis. 10 (1): 127–136. doi:10.1002/dta.2228. PMID 28608586.
23. Nordmeier F, Cannaert A, Stove CP, Schmidt PH, Meyer MR, Schaefer N (April 2022). “Are the N-demethylated metabolites of U-47700 more active than their parent compound? In vitro μ-opioid receptor activation of N-desmethyl-U-47700 and N,N-bisdesmethyl-U-47700”. Drug Testing and Analysis. 14 (4): 713–717. doi:10.1002/dta.3182. hdl:1854/LU-8751266. PMID 34669261.
24. Truver MT, Smith CR, Garibay N, Kopajtic TA, Swortwood MJ, Baumann MH (October 2020). “Pharmacodynamics and pharmacokinetics of the novel synthetic opioid, U-47700, in male rats”. Neuropharmacology. 177: 108195. doi:10.1016/j.neuropharm.2020.108195. PMC 7554234. PMID 32533977. S2CID 219559700.
25. Davis TL (9 March 2016). “New Synthetic Opioid Surfaces in North Texas”. NBCDFW.
26. Jones MJ, Hernandez BS, Janis GC, Stellpflug SJ (January 2017). “A case of U-47700 overdose with laboratory confirmation and metabolite identification”. Clinical Toxicology. 55 (1): 55–59. doi:10.1080/15563650.2016.1209767. PMID 27549165. S2CID 27920117.
27. Domanski K, Kleinschmidt KC, Schulte JM, Fleming S, Frazee C, Menendez A, Tavakoli K (January 2017). “Two cases of intoxication with new synthetic opioid, U-47700”. Clinical Toxicology. 55 (1): 46–50. doi:10.1080/15563650.2016.1209763. PMID 27432224. S2CID 46228909.
28. Ruan X, Chiravuri S, Kaye AD (September 2016). “Comparing fatal cases involving U-47700”. Forensic Science, Medicine, and Pathology. 12 (3): 369–371. doi:10.1007/s12024-016-9795-8. PMID 27421264. S2CID 24201474.
29. Elliott SP, Brandt SD, Smith C (August 2016). “The first reported fatality associated with the synthetic opioid 3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide (U-47700) and implications for forensic analysis”. Drug Testing and Analysis. 8 (8): 875–879. doi:10.1002/dta.1984. PMID 27232154. S2CID 205762837.
30. Armenian P, Olson A, Anaya A, Kurtz A, Ruegner R, Gerona RR (January 2017). “Fentanyl and a Novel Synthetic Opioid U-47700 Masquerading as Street “Norco” in Central California: A Case Report”. Annals of Emergency Medicine. 69 (1): 87–90. doi:10.1016/j.annemergmed.2016.06.014. PMID 27473610.
31. Helander A, Bäckberg M (January 2017). “New Psychoactive Substances (NPS) – the Hydra monster of recreational drugs”. Clinical Toxicology. 55 (1): 1–3. doi:10.1080/15563650.2016.1217003. PMID 27549399. S2CID 35645218.
32. Rambaran KA, Fleming SW, An J, Burkhart S, Furmaga J (October 2017). “U-47700: A Clinical Review of the Literature”. The Journal of Emergency Medicine. 53 (4): 509–519. doi:10.1016/j.jemermed.2017.05.034. PMID 28911989.
33. Kimergård A, Breindahl T, Hindersson P, Deluca P (October 2016). “Tampering of opioid analgesics: a serious challenge for public health?”. Addiction. 111 (10): 1701–1702. doi:10.1111/add.13436. PMID 27273814.
34. Coopman V, Blanckaert P, Van Parys G, Van Calenbergh S, Cordonnier J (September 2016). “A case of acute intoxication due to combined use of fentanyl and 3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide (U-47700)”. Forensic Science International. 266: 68–72. doi:10.1016/j.forsciint.2016.05.001. hdl:1854/LU-8509152. PMID 27235591.
35. “Twee doden in België door overdosis met fentanylpleisters” (in Dutch). Deredactie. 29 January 2016.
36. Koch K, Auwärter V, Hermanns-Clausen M, Wilde M, Neukamm MA (April 2018). “Mixed intoxication by the synthetic opioid U-47700 and the benzodiazepine flubromazepam with lethal outcome: Pharmacokinetic data”. Drug Testing and Analysis. 10 (8): 1336–1341. doi:10.1002/dta.2391. PMID 29637722.
37. “Young people playing Russian roulette with drugs, warns coroner”. The Irish Times.
38. “Torino, ucciso dalla droga comprata online: la prima vittima italiana dell’U47700”. LaStampa.it (in Italian). Retrieved 2017-10-20.
39. Zalkind S (11 April 2016). “Synthetic opiate makers stay step ahead of US drug laws as overdose cases rise”. The Guardian. ISSN 0261-3077.
40. Draper B (6 June 2016). “New synthetic drug U-47700 has states rushing to stop spread”. Associated Press.
41. Schneir A, Metushi IG, Sloane C, Benaron DJ, Fitzgerald RL (January 2017). “Near death from a novel synthetic opioid labeled U-47700: emergence of a new opioid class”. Clinical Toxicology. 55 (1): 51–54. doi:10.1080/15563650.2016.1209764. PMID 27448790. S2CID 25206275.
42. Mohr AL, Friscia M, Papsun D, Kacinko SL, Buzby D, Logan BK (November 2016). “Analysis of Novel Synthetic Opioids U-47700, U-50488 and Furanyl Fentanyl by LC-MS/TS in Postmortem Casework”. Journal of Analytical Toxicology. 40 (9): 709–717. doi:10.1093/jat/bkw086. PMID 27590036.
43. “19 Recent Deaths Associated With Synthetic Opioids; State Officials Urge Awareness”. NC Department of Health and Human Services. 24 March 2016.
44. Drug Enforcement Administration (DEA) (7 September 2016). “Proposed Rule: Schedules of Controlled Substances: Temporary Placement of U-47700 Into Schedule I”. Federal Register.
45. Krotulski AJ, Mohr AL, Papsun DM, Logan BK (January 2018). “Metabolism of novel opioid agonists U-47700 and U-49900 using human liver microsomes with confirmation in authentic urine specimens from drug users”. Drug Testing and Analysis. 10 (1): 127–136. doi:10.1002/dta.2228. PMID 28608586.
46. Moody MT, Diaz S, Shah P, Papsun D, Logan BK (September 2018). “Analysis of fentanyl analogs and novel synthetic opioids in blood, serum/plasma, and urine in forensic casework”. Drug Testing and Analysis. 10 (9): 1358–1367. doi:10.1002/dta.2393. PMID 29633785. S2CID 4758125.
47. “Furanyl Fentanyl Joins U-47,700 As The Second Illicit Opioid Banned By DEA In November”. Forbes.
48. Baselt, R. (2017) Disposition of Toxic Drugs and Chemicals in Man, 11th edition, Biomedical Publications, Foster City, CA, pp. 2208.
49. “Pinky”. pubchem.ncbi.nlm.nih.gov. National Library of Medicine. Retrieved 16 July 2020.
50. “31 nya ämnen kan klassas som narkotika eller hälsofarlig vara” (in Swedish). Folkhälsomyndigheten. November 2015.
51. Kasich JR (May 3, 2016). “Executive Order 2016-01K” (PDF). Governor of Ohio.
52. “News Release – Attorney General Bondi Outlaws Deadly Synthetic Drug”. www.myfloridalegal.com. Retrieved 2016-09-27.
53. DEA Public Affairs (November 11, 2016). “46 confirmed deaths linked to dangerous opioid in ’15 and ’16 spark emergency action”. DEA.