Summary
2,5-Dimethoxy-4-ethylamphetamine, also known as DOET, DOE, or Hecate, belongs to the psychedelic realm of drugs within the phenethylamine and amphetamine chemical categories. The compound was initially created by Alexander Shulgin and was documented in his literary work, PiHKAL (Phenethylamines I Have Known And Loved).
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 22004-32-6 |
|---|---|
| PubChem CID | 27402 |
| DrugBank | DB01467 |
| ChemSpider | 25499 |
| UNII | 9SK6K682UL |
| ChEMBL | ChEMBL8224 |
| Chemical and physical data | |
| Formula | C13H21NO2 |
| Molar mass | 223.316 g·mol−1 |
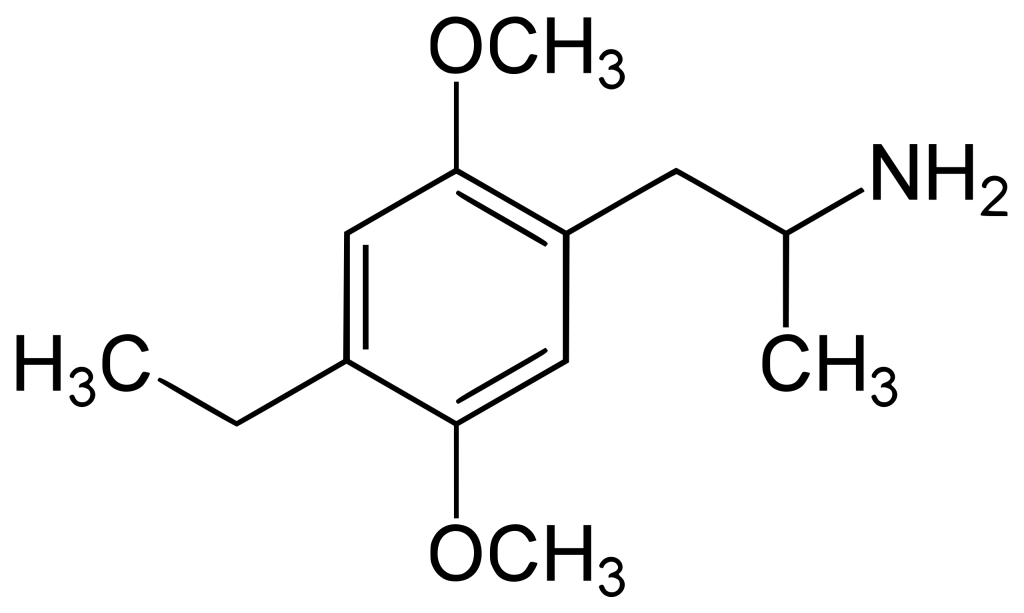
Chemistry
DOET belongs to a group of substances widely recognized as substituted amphetamines. Its chemical denomination is 4-ethyl-2,5-dimethoxy-alpha-methylbenzeneethanamine or 1-(2,5-dimethoxy-4-ethylphenyl)propan-2-amine. The (R)-DOET enantiomer is more active with an active stereocenter. Despite being an exceptionally uncommon compound, information on its effects and toxicity in humans is scarce. Nevertheless, akin to the more prevalent 2,5-dimethoxy-amphetamine analogs like DOB, DOI, and DOM, DOET demonstrates potent and enduring psychedelic properties. When the alpha-methyl group is eliminated, the resulting compound, 2C-E, represents another psychedelic substance initially synthesized by Dr. Alexander Shulgin.
Pharmacology
Much like its counterparts such as DOM, DOET presumably operates as a partial agonist of the 5-HT2A, 5-HT2B, and 5-HT2C receptors (citation needed). Additionally, it serves as an agonist of the human TAAR1 receptor.
Effects
The psychedelic impact of DOET is characterized by an extended duration, lasting up to 14–20 hours. Within PiHKAL, Shulgin denotes the recommended oral dosage of DOET to be 2–7 mg, with 6–7 mg being the dose necessary for experiencing the complete and desired effects.
Legal status
Globally, DOET is categorized as a Schedule I controlled substance, restricting its use to medicinal purposes and scientific investigation under the Convention on Psychotropic Substances regulations.
In the United States, DOET is classified as a Schedule I substance and shares a similar controlled status in various other regions worldwide.
In Australia, DOET is designated as a Schedule 9 prohibited substance according to the Poisons Standard (October 2015). A Schedule 9 substance is prone to misuse or abuse, necessitating its prohibition for manufacturing, possession, sale, or use, except for authorized medical or scientific research or analytical, educational, or training intentions with the endorsement of the appropriate Commonwealth and State or Territory Health Authorities.
FAQ
- What is 2,5-Dimethoxy-4-ethylamphetamine (DOET)?
- 2,5-Dimethoxy-4-ethylamphetamine (DOET) is a psychedelic compound that falls within the phenethylamine and amphetamine classes of chemicals. It is known for its potent and long-lasting psychedelic effects.
- Who discovered DOET?
- DOET was first synthesized and documented by Alexander Shulgin, a renowned American medicinal chemist and pharmacologist. He detailed the compound in his influential book “PiHKAL: A Chemical Love Story.”
- What are the effects of DOET?
- DOET is recognized for producing profound psychedelic effects lasting 14–20 hours. It is reported to induce altered perceptions, hallucinations, and sensory distortions.
- What is the recommended dosage for DOET?
- According to Shulgin’s research in “PiHKAL,” the recommended oral dosage of DOET ranges from 2 to 7 mg, with 6 to 7 mg being the dose for achieving the desired psychedelic effects.
- How is DOET regulated internationally?
- Internationally, DOET is classified as a Schedule I controlled substance under the Convention on Psychotropic Substances, limiting its use to medical and scientific research purposes.
- What is the legal status of DOET in the United States and Australia?
- In the United States, DOET is classified as a Schedule I substance, indicating it has a high potential for abuse and no accepted medical use. Similarly, it is designated as a Schedule 9 prohibited substance under the Poisons Standard in Australia.
- Are there any known risks or side effects of using DOET?
- Limited information is available on the specific risks and side effects of DOET due to its rarity. However, like other potent psychedelics, it may potentially induce adverse psychological effects and pose risks to individuals with pre-existing mental health conditions.
- Is DOET used for any legitimate medical purposes?
- DOET is not commonly used for legitimate medical purposes, primarily due to its potent psychedelic properties and associated risks. Research on its potential therapeutic applications is limited.
References
- Anvisa (2023-07-24): “RDC Nº 804 – Compilation of Substances with Sedative, Psychedelic, Precursor, and Other Exceptional Control” [Collegiate Board Resolution No. 804 – Inventories of Narcotic, Psychotropic, Precursor, and Other Materials under Unique Supervision] (in Brazilian Portuguese). Published in the Diário Oficial da União on 2023-07-25. The original document is archived until 2023-08-27, and the retrieval was conducted on 2023-08-27.
- Shulgin A, Shulgin A (September 1991). “PiHKAL: A Chemical Romance.” Published in the United States by Transform Press, encompassing page 978. The ISBN for this work is 0-9630096-0-5.
- Lewin AH, Miller GM, Gilmour B (December 2011). “Trace amine-associated receptor 1 is a stereospecific binding site for substances in the amphetamine family.” Published in Bioorganic & Medicinal Chemistry, volume 19, issue 23, pages 7044–7048. doi:10.1016/j.bmc.2011.10.007. The publication is available through PMC 3236098, and the PMID is 22037049.
- “Compound ID: CHEMBL2360469.” Sourced from ChEMBL and retrieved on 29 April 2014.
- “Poisons Standard.” Released by the Therapeutic Goods Administration, Australian Government, in October 2015.