Summary
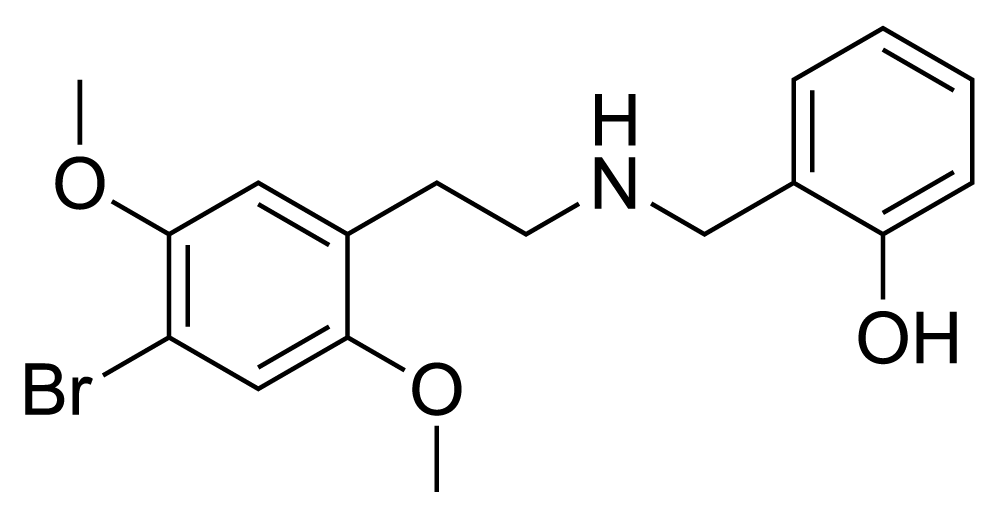
25B-NBOH, also recognized as 2C-B-NBOH or NBOH-2C-B, is a derivative stemming from the phenethylamine family of hallucinogenic substances, specifically derived from 2C-B. It has been marketed as a designer drug. Functionally, 25B-NBOH acts as a potent serotonin receptor agonist, exhibiting a similar affinity to the more widely known compound, 25B-NBOMe, at both the 5-HT2A and 5-HT2C receptors. It achieves this with pKi values, although specific clarification is needed, that measure 8.3 and 9.4, respectively.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1335331-46-8 |
|---|---|
| PubChem CID | 125181394 |
| ChemSpider | 58191433 |
| UNII | KHR1SJ9L0Y |
| Chemical and physical data | |
| Formula | C17H20BrNO3 |
| Molar mass | 366.255 g·mol−1 |
Legal status
Sweden:
On January 26, 2016, the Riksdag incorporated 25B-NBOH into the Narcotic Drugs Punishments Act, designating it under Swedish Schedule I, which encompasses “substances, plant materials, and fungi typically devoid of medical utility.” This classification was documented by the Medical Products Agency (MPA) within regulation HSLF-FS 2015:35, listing it as 25B-NBOH, and 2-([2-(4-bromo-2,5-dimethoxyphenyl)ethylamino]methyl)phenol.
United Kingdom:
In the United Kingdom, this substance is classified as a Class A drug due to its inclusion in the N-benzyl phenethylamine catch-all clause within the Misuse of Drugs Act of 1971.
FAQ
- What is 25B-NBOH? 25B-NBOH, also known as 2C-B-NBOH or NBOH-2C-B, is a derivative of the phenethylamine family, derived from the hallucinogenic substance 2C-B.
- What Are the Effects of 25B-NBOH? 25B-NBOH acts as a potent serotonin receptor agonist, potentially causing hallucinogenic effects, altered perception, and changes in consciousness.
- Is 25B-NBOH Legal in Sweden? No, in Sweden, 25B-NBOH is classified as a controlled substance under Schedule I, a category that includes substances typically considered to have no medical use.
- Is 25B-NBOH Legal in the United Kingdom? No, in the United Kingdom, 25B-NBOH is categorized as a Class A drug, governed by the Misuse of Drugs Act 1971.
- What Are the Potential Risks of Using 25B-NBOH? The use of 25B-NBOH may entail risks, including hallucinations and psychological effects. It’s essential to exercise caution and be aware of potential side effects.
- Is 25B-NBOH Used for Any Therapeutic Purposes? There is no recognized therapeutic use for 25B-NBOH, and it is primarily known for its recreational and hallucinogenic effects.
- Where Can I Find More Information on 25B-NBOH? You can explore scientific literature, online resources, and forums for additional information about 25B-NBOH. However, always prioritize safety and be aware of the legal status in your area before considering its use.
References
- Anvisa (2023-07-24). “Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control” (in Brazilian Portuguese). Published in the Diário Oficial da União on 2023-07-25. Archived from the original on 2023-08-27. Retrieved on 2023-08-27.
- Heim R (19 March 2004). “Synthesis and Pharmacology of Potent 5-HT2A Receptor Agonists with N-2-Methoxybenzyl Partial Structure” (Ph.D. thesis) pursued at Freie Universität Berlin. Archived from the original on 16 April 2012. Retrieved on 27 June 2015.
- Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL (March 2014). “Elucidating the Synthesis and Structure-Activity Relationships of N-Benzyl Phenethylamines as 5-HT2A/2C Agonists” in ACS Chemical Neuroscience, Volume 5, Issue 3, Pages 243–249. doi:10.1021/cn400216u. PMC 3963123. PMID 24397362.
- Hansen M (2010-12-16). “Innovative Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain” (Ph.D. thesis) pursued at the University of Copenhagen. doi:10.13140/RG.2.2.33671.14245.
- Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, et al. (April 2011). “Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers” in the European Journal of Nuclear Medicine and Molecular Imaging, Volume 38, Issue 4, Pages 681–693. doi:10.1007/s00259-010-1686-8. PMID 21174090.
- Silva ME, Heim R, Strasser A, Elz S, Dove S (January 2011). “Theoretical studies on the interaction of partial agonists with the 5-HT2A receptor” in the Journal of Computer-Aided Molecular Design, Volume 25, Issue 1, Pages 51–66. doi:10.1007/s10822-010-9400-2. PMID 21088982.
- Braden MR, Parrish JC, Naylor JC, Nichols DE (December 2006). “Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists” in Molecular Pharmacology, Volume 70, Issue 6, Pages 1956–1964. doi:10.1124/mol.106.028720. PMID 17000863.
- “The Swedish Medicines Agency’s Regulations on Amendments to the Swedish Medicines Agency’s Regulations (LVFS 2011: 10) on Lists of Drugs” (PDF) – Läkemedelsverket.
- “The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014” – Available at www.legislation.gov.uk.
- “Explore N-(2C-B)-Fentanyl” | Accessible via PiHKAL · info on isomerdesign.com.
- “Explore N-(2C-FLY)-Fentanyl” | Accessible via PiHKAL · info on isomerdesign.com.
- Glennon, Richard A.; Bondarev, Mikhail L.; Khorana, Nantaka; Young, Richard; May, Jesse A.; Hellberg, Mark R.; McLaughlin, Marsha A.; Sharif, Najam A. (November 2004). “Exploring β-Oxygenated Analogues of the 5-HT2A Serotonin Receptor Agonist 1-(4-Bromo-2,5-Dimethoxyphenyl)-2-Aminopropane” in the Journal of Medicinal Chemistry, Volume 47, Issue 24, Pages 6034–6041. doi:10.1021/jm040082s. PMID 15537358.
- “Beta-Hydroxyphenylalkylamines and Their Potential Use for Treating Glaucoma.”