Summary
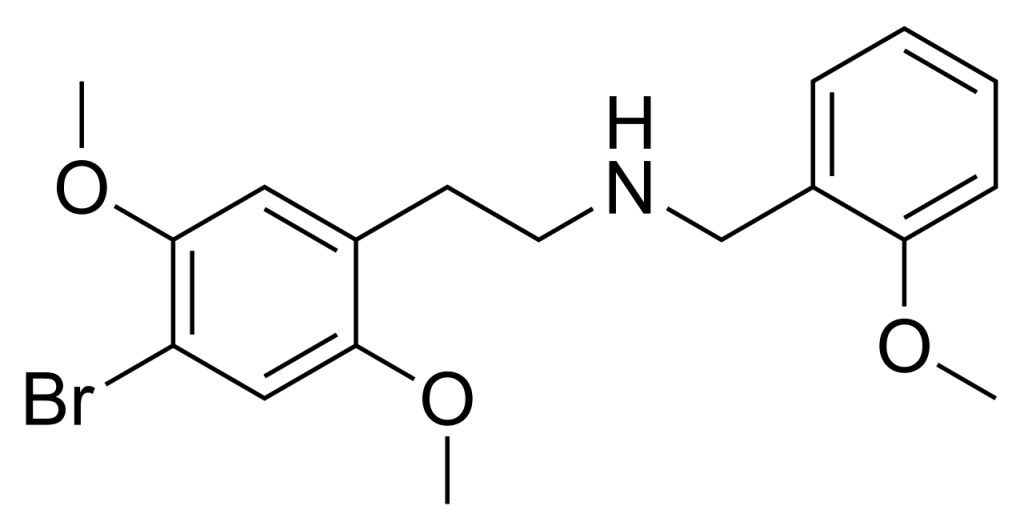
25B-NBOMe, also known as NBOMe-2C-B, Cimbi-36, Nova, or BOM 2-CB, belongs to the phenethylamine psychedelic class and was initially discovered in 2004 by Ralf Heim at the Free University of Berlin. This compound functions as a potent full agonist for the 5HT2A receptor.
Anecdotal reports from users have suggested that 25B-NBOMe can induce hallucinogenic effects at doses as low as 250–500 µg, demonstrating similar potency to other phenethylamine-derived hallucinogens like Bromo-DragonFLY. The duration of its effects is reported to last approximately 12–16 hours. However, when used in the radiolabeled form for tracer doses, the parent compound is swiftly eliminated from the bloodstream.
Recent research conducted by Custodio et al. in 2019 examined the potential involvement of a dysregulated dopaminergic system, neuroadaptation, and changes in brain waves that might contribute to the rewarding and reinforcing properties of 25B-NBOMe in rodents.
A carbon-11 labelled version of this compound, [11C]Cimbi-36, was synthesized and validated as a radioactive tracer for positron emission tomography (PET) in Copenhagen. As a 5-HT2A receptor agonist PET radioligand, [11C]Cimbi-36 was believed to serve as a functional marker of these receptors and a potential indicator of serotonin release, allowing for the assessment of serotonin levels in vivo. Clinical trials involving [11C]Cimbi-36 as a PET ligand in humans are currently underway.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1026511-90-9 |
|---|---|
| PubChem CID | 9977044 |
| ChemSpider | 8152636 |
| UNII | S6NAA81PHK |
| CompTox Dashboard (EPA) | DTXSID50907974 |
| Chemical and physical data | |
| Formula | C18H22BrNO3 |
| Molar mass | 380.282 g·mol−1 |
Toxicity and harm potential
NBOMe compounds are frequently linked to severe toxicity and even fatal outcomes. Extensive research on the NBOMe family of compounds has revealed their potential for neurotoxic and cardiotoxic effects. Individuals who have encountered NBOMe compounds have commonly reported symptoms of autonomic dysfunction, including sympathomimetic toxicity, such as vasoconstriction, hypertension, and tachycardia, alongside hallucinations. These symptoms may be accompanied by restlessness, seizures, hyperthermia, profuse sweating, muscle rigidity, rhabdomyolysis, and, tragically, fatalities. Researchers have noted that intoxication with NBOMe often manifests signs of serotonin syndrome. Additionally, the likelihood of experiencing seizures is higher with NBOMes when compared to other psychedelics.
A concerning aspect is that NBOMe and NBOH compounds are sometimes deceitfully marketed as LSD, even sold on blotter papers. This misrepresentation is problematic because they differ significantly in terms of taste and safety profiles from genuine LSD. Despite their high potency, recreational doses of LSD have generally resulted in few cases of acute toxicity. Regrettably, fatalities attributed to NBOMe intoxication underscore that many individuals may have ingested the substance, believing it to be LSD. Researchers have also pointed out that individuals familiar with LSD may mistakenly assume NBOMe is equally safe. Tragically, apart from physical effects, there have been instances of self-harm and suicide linked to NBOMe use.
Due to limited documentation on NBOMe consumption, the long-term effects of these substances still need to be discovered. It’s worth noting that NBOMe compounds are not active when ingested orally, and they are typically consumed sublingually.: 3 Sublingual administration may lead to numbness of the tongue and mouth, followed by a distinctive metallic chemical taste. Researchers have identified this physical side effect as a key distinguishing factor between NBOMe compounds and LSD.
Neurotoxic and Cardiotoxic Actions:
Many NBOMe compounds exhibit high-potency agonist activity at various 5-HT receptors, and prolonged stimulation of the 5-HT2B receptor, in particular, can lead to cardiac valvulopathy, especially at high doses and with chronic use. Research has strongly associated 5-HT2B receptors with drug-induced valvular heart disease. The high affinity of NBOMe compounds for adrenergic α1 receptors contributes to their stimulant-like cardiovascular effects.
In laboratory studies, 25C-NBOMe demonstrated cytotoxicity in neuronal cell lines SH-SY5Y, PC12, and SN471. It was more potent than methamphetamine in reducing cell viability, with neurotoxicity involving the activation of the MAPK/ERK cascade and inhibition of the Akt/PKB signalling pathway. Furthermore, 25C-NBOMe, along with its derivative 25D-NBOMe, reduced the viability of cardiomyocytes H9c2 cells and downregulated the expression of p21 (CDC24/RAC)-activated kinase 1 (PAK1), an enzyme known for its cardiac protective effects.
Preliminary studies involving 25C-NBOMe have indicated toxicity to development, heart health, and brain health in zebrafish, rats, and Artemia salina. Further research is needed to determine the applicability of these toxicology results to humans and to assess the impact of the substance on pregnant women and their developing fetuses.
Emergency Treatment:
Currently, there are no specific antidotes for NBOMes, and the management of acute intoxication relies on symptomatic treatments. These may include the administration of benzodiazepines, antipsychotic medications, and antiarrhythmic agents like beta blockers. Some emergency interventions are tailored to address rhabdomyolysis, a condition that can lead to critical complications such as metabolic acidosis and acute kidney injury.
Legal status
Canada:
As of October 31, 2016, 25B-NBOMe is categorized as a controlled substance (Schedule III) in Canada.
Russia:
Since May 5, 2015, 25B-NBOMe has been classified as a banned narcotic drug in Russia.
Sweden:
In Sweden, 25B-NBOMe was included in schedule I, encompassing substances, plant materials, and fungi typically devoid of medical use. This classification was implemented on August 1, 2013, and published by the Medical Products Agency under regulation LVFS 2013:15, listing it as 25B-NBOMe, which is 2-(4-bromo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine.
United Kingdom:
In the United Kingdom, this substance is categorized as a Class A drug due to its inclusion in the N-benzyl phenethylamine catch-all clause in the Misuse of Drugs Act 1971.
United States:
In November 2013, the U.S. Drug Enforcement Administration designated 25B-NBOMe (alongside 25I-NBOMe and 25C-NBOMe) as Schedule I substances under the Controlled Substances Act, rendering their manufacture, purchase, possession, processing, and distribution illegal.
China:
Since October 2015, 25B-NBOMe has been classified as a controlled substance in China.
Czech Republic:
The Czech Republic has also prohibited the use of 25B-NBOMe.
FAQ
- What is 25B-NBOMe?
- 25B-NBOMe is a synthetic hallucinogenic substance belonging to the NBOMe family. It is chemically related to the phenethylamine 2C-B and is known for its hallucinogenic properties.
- How is 25B-NBOMe typically consumed?
- 25B-NBOMe is often taken sublingually (under the tongue), where it can be absorbed into the bloodstream. It is essential to note that it is not active when ingested orally.
- What are the effects of 25B-NBOMe?
- 25B-NBOMe is known for inducing hallucinations and altering perception. Users have reported visual distortions, changes in thought processes, and sometimes intense emotional experiences. These effects can be unpredictable and vary between individuals.
- What are the risks associated with 25B-NBOMe?
- 25B-NBOMe is associated with potentially severe risks, including life-threatening toxicity and fatalities. Some individuals have experienced autonomic dysfunction, sympathomimetic toxicity (vasoconstriction, hypertension, tachycardia), and even seizures.
- Is 25B-NBOMe legal?
- The legal status of 25B-NBOMe varies by country. It is classified as a controlled substance in many places, and its possession, distribution, or use may be illegal.
- What distinguishes 25B-NBOMe from other hallucinogenic substances?
- 25B-NBOMe is chemically distinct from other hallucinogens and is known for causing numbing of the tongue and mouth when taken sublingually. This characteristic taste and sensation can help differentiate it from other substances like LSD.
- Can 25B-NBOMe be confused with other drugs?
- Yes, 25B-NBOMe is sometimes misrepresented as LSD. Blotter papers containing 25B-NBOMe can have a bitter taste, unlike the typically tasteless or slightly metallic taste of LSD.
- Are there any long-term effects of 25B-NBOMe use?
- Due to limited documentation, the long-term effects of 25B-NBOMe use need to be better understood. Research on this topic is ongoing.
- Is there emergency treatment available for 25B-NBOMe intoxication?
- Currently, there are no specific antidotes for 25B-NBOMe. Acute intoxication is managed through symptomatic treatments, which may include benzodiazepines, antipsychotic medications, and antiarrhythmic agents.
- Is 25B-NBOMe still available on the market?
- The availability of 25B-NBOMe may vary by location and over time. However, it is important to note that its use may be illegal in many jurisdictions due to safety concerns. It is strongly discouraged to use unregulated substances.
References
- Anvisa (July 24, 2023). “RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” [Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published July 25, 2023). Archived from the original on August 27, 2023. Retrieved August 27, 2023.
- Heim R (February 28, 2010). “Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur. Entwicklung eines neuen Struktur-Wirkungskonzepts” (in German). diss.fu-berlin.de. Retrieved May 10, 2013.
- Silva M (2009). Theoretical study of the interaction of agonists with the 5-HT2A receptor (Ph.D. thesis). Universität Regensburg.
- Silva ME, Heim R, Strasser A, Elz S, Dove S (January 2011). “Theoretical studies on the interaction of partial agonists with the 5-HT2A receptor”. Journal of Computer-Aided Molecular Design. 25 (1): 51–66. doi:10.1007/s10822-010-9400-2.
- Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL (March 2014). “Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists”. ACS Chemical Neuroscience. 5 (3): 243–9.
- Ettrup A, da Cunha-Bang S, McMahon B, Lehel S, Dyssegaard A, Skibsted AW, et al. (July 2014). “Serotonin 2A receptor agonist binding in the human brain with [¹¹C]Cimbi-36”. Journal of Cerebral Blood Flow and Metabolism. 34 (7): 1188–96.
- Custodio RJ, Sayson LV, Botanas CJ, Abiero A, You KY, Kim M, et al. (November 2020). “25B-NBOMe, a novel N-2-methoxybenzyl-phenethylamine (NBOMe) derivative, may induce rewarding and reinforcing effects via a dopaminergic mechanism: Evidence of abuse potential”. Addiction Biology. 25 (6): e12850.
- Hansen M (December 16, 2010). Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain (Ph.D. thesis). University of Copenhagen.
- Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, Lehel S, Herth MM, Madsen J, et al. (April 2011). “Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers”. European Journal of Nuclear Medicine and Molecular Imaging. 38 (4): 681–93.
- Ettrup A, Holm S, Hansen M, Wasim M, Santini MA, Palner M, et al. (August 2013). “Preclinical safety assessment of the 5-HT2A receptor agonist PET radioligand [ 11C]Cimbi-36”. Molecular Imaging and Biology. 15 (4): 376–383.
- Johansen A, Hansen HD, Svarer C, Lehel S, Leth-Petersen S, Kristensen JL, et al. (April 2018). “The importance of small polar radiometabolites in molecular neuroimaging: A PET study with [11C]Cimbi-36 labeled in two positions”.
- Johansen A, Holm S, Dall B, Keller S, Kristensen JL, Knudsen GM, Hansen HD (July 2019). “Human biodistribution and radiation dosimetry of the 5-HT2A receptor agonist Cimbi-36 labeled with carbon-11 in two positions”.
- “From molecule to man: The full CIMBI-36 story” (PDF). cimbi.dk.
- “Imanova announces the launch of a new imaging biomarker to investigate the serotonin system in psychiatric illness”. imanova.co.uk. Archived from the original on April 9, 2015.
- Madsen MK, Fisher PM, Burmester D, Dyssegaard A, Stenbæk DS, Kristiansen S, et al. (June 2019). “Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels”.
- Sean I, Joe R, Jennifer S, and Shaun G (March 28, 2022). “A cluster of 25B-NBOH poisonings following exposure to powder sold as lysergic acid diethylamide (LSD)”. Clinical Toxicology. 60 (8): 966–969.
- Amy E, Katherine W, John R, Sonyoung K, Robert J, Aaron J (December 2018). “Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors”.
- Jolanta Z, Monika K, and Piotr A (February 26, 2020). “NBOMes–Highly Potent and Toxic Alternatives of LSD”.
- Lipow M, Kaleem SZ, Espiridion E (March 30, 2022). “NBOMe Toxicity and Fatalities: A Review of the Literature”.