Summary
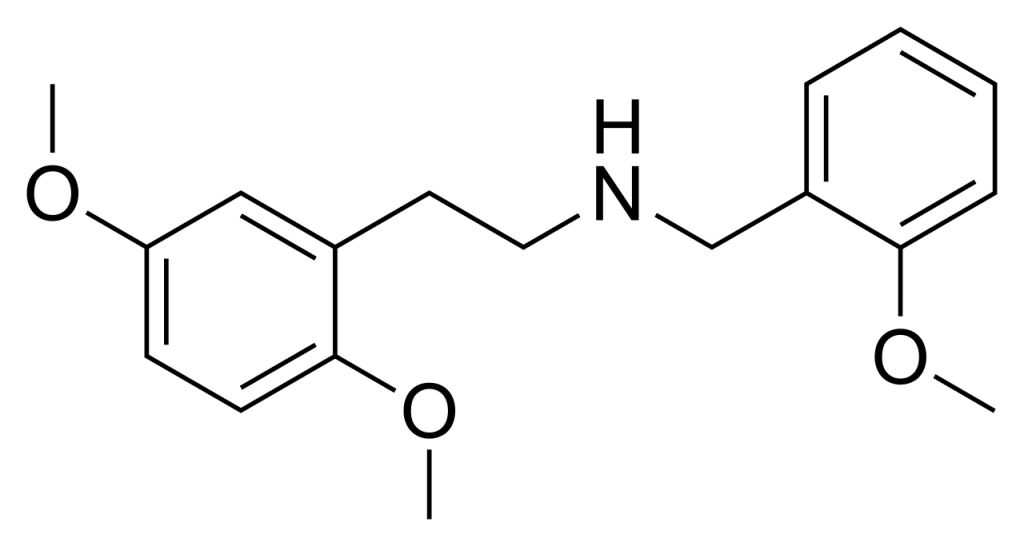
25H-NBOMe (also known as NBOMe-2C-H) is a compound derived from the phenethylamine hallucinogen 2C-H. It functions as a remarkably potent full agonist targeting the human 5-HT2A receptor.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 919797-16-3 |
|---|---|
| PubChem CID | 39424372 |
| ChemSpider | 25511924 |
| UNII | RN5ZVN74Y9 |
| Chemical and physical data | |
| Formula | C18H23NO3 |
| Molar mass | 301.386 g·mol−1 |
Toxicity
NBOMe substances are frequently linked to severe toxicity and fatalities. Research on the NBOMe compound family has revealed their neurotoxic and cardiotoxic properties. Autonomic dysfunction, including vasoconstriction, hypertension, and tachycardia, is familiar with NBOMe compounds, often accompanied by hallucinations. Additionally, symptoms of toxidrome include agitation, seizure, hyperthermia, diaphoresis, hypertonia, rhabdomyolysis, and even death. Incidents of NBOMe intoxication often manifest signs of serotonin syndrome, with a higher likelihood of seizures than other psychedelics.
Sold as LSD on blotter papers, NBOMe and NBOH compounds can have a bitter taste and distinct safety profiles. Despite their high potency, recreational doses of LSD have shown minimal incidents of acute toxicity. Fatalities related to NBOMe ingestion suggest that many individuals unknowingly consumed the substance, believing it to be LSD, leading researchers to caution that “users familiar with LSD may mistakenly assume safety when ingesting NBOMe.”[6] While most deaths are attributed to the physical effects of the drug, there have been reports of fatalities due to self-harm and suicide under its influence.
Due to the limited documentation of NBOMe consumption, the long-term effects of the substance still need to be clarified. NBOMe compounds are not active orally[a] and are typically taken sublingually.: 3 Sublingual administration of NBOMes often results in tongue and mouth numbness followed by a metallic chemical taste, considered a critical distinguishing feature between NBOMe compounds and LSD by researchers.
Legality
Sweden:
In Sweden, 25H-NBOMe was included in the Narcotic Drugs Punishments Act under Swedish Schedule I (“substances, plant materials, and fungi which normally do not have medical use”) effective August 1, 2013. This edition was published by the Medical Products Agency (MPA) in regulation LVFS 2013:15, listed as 25H-NBOMe, and 2-(2,5-methoxyphenyl)-N-(2-methoxybenzyl)ethanamine.
United Kingdom:
The United Kingdom categorizes this substance as a Class A drug under the N-benzyl phenethylamine catch-all clause in the Misuse of Drugs Act 1971.
FAQ
- What is 25H-NBOMe?
- 25H-NBOMe is a synthetic psychedelic compound that belongs to the NBOMe family. It is a potent agonist for the 5-HT2A receptor, the same receptor targeted by classic psychedelics like LSD and psilocybin.
- How is 25H-NBOMe used?
- 25H-NBOMe is typically used by placing a blotter paper containing the compound under the tongue (sublingually) or swallowing it. The sublingual method is more common, and the blotter is allowed to dissolve in the mouth.
- What are the effects of 25H-NBOMe?
- The effects of 25H-NBOMe can include altered perceptions, hallucinations, changes in mood, and an enhanced sensory experience. However, it’s important to note that these effects can vary widely between individuals.
- Is 25H-NBOMe safe to use?
- 25H-NBOMe is associated with significant risks, including life-threatening toxicity. It has been linked to cases of severe adverse effects and even fatalities. Using 25H-NBOMe can be dangerous, and its safety profile is a subject of concern.
- What are the risks of 25H-NBOMe use?
- Using 25H-NBOMe is associated with various risks, including neurotoxic and cardiotoxic effects. Users may experience symptoms such as vasoconstriction, hypertension, tachycardia, hallucinations, etc. There’s also a higher risk of seizure compared to other psychedelics.
- Is 25H-NBOMe legal?
- The legal status of 25H-NBOMe varies by country and region. It is controlled or banned in some places due to its potential risks. Users should be aware of and comply with their local drug laws.
- How can I stay safe if I choose to use 25H-NBOMe?
- It is not recommended to use 25H-NBOMe due to its associated risks. If you decide to use it, it is crucial to prioritize safety. Start with a low dose, be in a safe and comfortable environment, and have a trusted friend present. If you experience adverse effects, seek medical attention immediately.
- Is there an antidote for 25H-NBOMe intoxication?
- At present, there is no specific antidote for 25H-NBOMe intoxication. Management of acute intoxication typically involves symptomatic treatments to address specific symptoms or complications.
- What should I do if I suspect someone is experiencing an adverse reaction to 25H-NBOMe?
- If someone appears to be experiencing severe or unusual symptoms after using 25H-NBOMe, it is essential to seek medical help immediately. The person should be taken to the nearest emergency room for evaluation and treatment.
- Where can I find more information about 25H-NBOMe?
- For more detailed information about 25H-NBOMe, you can consult scientific literature, toxicology reports, and reliable online sources. However, always exercise caution and prioritize safety if considering its use.
References
- Anvisa (2023-07-24). “RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” [Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- Amy E, Katherine W, John R, Sonyoung K, Robert J, Aaron J (December 2018). “Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors”. Biochemical Pharmacology. 158: 27–34. doi:10.1016/j.bcp.2018.09.024. PMC 6298744. PMID 30261175.
- Sean I, Joe R, Jennifer S, and Shaun G (28 March 2022). “A cluster of 25B-NBOH poisonings following exposure to powder sold as lysergic acid diethylamide (LSD)”. Clinical Toxicology. 60 (8): 966–969. doi:10.1080/15563650.2022.2053150. PMID 35343858. S2CID 247764056.
- Jolanta Z, Monika K, and Piotr A (26 February 2020). “NBOMes–Highly Potent and Toxic Alternatives of LSD”. Frontiers in Neuroscience. 14: 78. doi:10.3389/fnins.2020.00078. PMC 7054380. PMID 32174803.
- Lipow M, Kaleem SZ, Espiridion E (30 March 2022). “NBOMe Toxicity and Fatalities: A Review of the Literature”. Transformative Medicine. 1 (1): 12–18. doi:10.54299/tmed/msot8578. ISSN 2831-8978. S2CID 247888583.
- Micaela T, Sabrine B, Raffaella A, Giorgia C, Beatrice M, Tatiana B, Federica B, Giovanni S, Francesco B, Fabio G, Krystyna G, Matteo M (21 April 2022). “Effect of -NBOMe Compounds on Sensorimotor, Motor, and Prepulse Inhibition Responses in Mice in Comparison With the 2C Analogs and Lysergic Acid Diethylamide: From Preclinical Evidence to Forensic Implication in Driving Under the Influence of Drugs”. Front Psychiatry. 13: 875722. doi:10.3389/fpsyt.2022.875722. PMC 9069068. PMID 35530025.
- Cristina M, Matteo M, Nicholas P, Maria C, Micaela T, Raffaella A, Maria L (12 December 2019). “Neurochemical and Behavioral Profiling in Male and Female Rats of the Psychedelic Agent 25I-NBOMe”. Frontiers in Pharmacology. 10: 1406. doi:10.3389/fphar.2019.01406. PMC 6921684. PMID 31915427.
- Anna R, Dino L, Julia R, Daniele B, Marius H, Matthias L (December 2015). “Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs)”. Neuropharmacology. 99: 546–553. doi:10.1016/j.neuropharm.2015.08.034. ISSN 1873-7064. PMID 26318099. S2CID 10382311.
- David W, Roumen S, Andrew C, Paul D (6 February 2015). “Prevalence of use and acute toxicity associated with the use of NBOMe drugs”. Clinical Toxicology. 53 (2): 85–92. doi:10.3109/15563650.2015.1004179. PMID 25658166. S2CID 25752763.
- Humston C, Miketic R, Moon K, Ma P, Tobias J (2017-06-05). “Toxic Leukoencephalopathy in a Teenager Caused by the Recreational Ingestion of 25I-NBOMe: A Case Report and Review of Literature”. Journal of Medical Cases. 8 (6): 174–179. doi:10.14740/jmc2811w. ISSN 1923-4163.