Summary
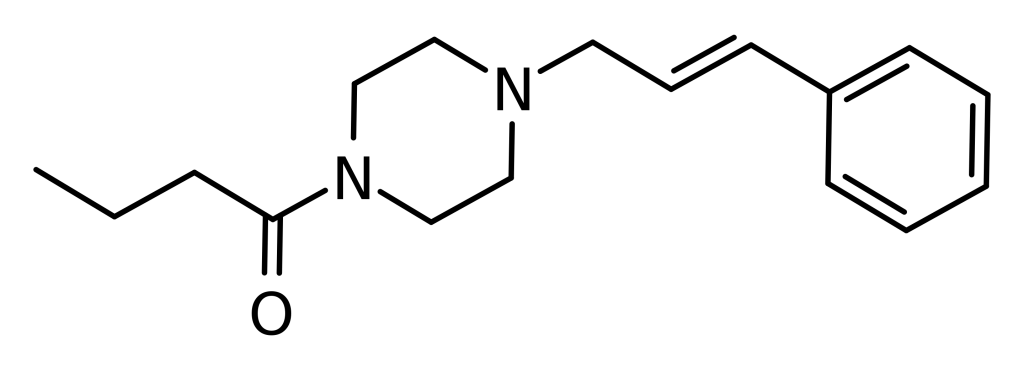
Bucinnazine, also known as AP-237 or 1-butyryl-4-cinnamylpiperazine, is an opioid analgesic drug with a significant history of use in China for managing pain in cancer patients dating back to 1986. This compound is among the most potent in a series of piperazine-amides that were initially synthesized and documented in Japan during the 1970s. Bucinnazine demonstrates analgesic potency comparable to morphine while maintaining a higher therapeutic index.
While the drug was initially touted as a non-narcotic analgesic, subsequent research has revealed that bucinnazine and similar acyl piperazines are powerful and selective agonists of the μ-opioid receptor (MOR), with relatively low affinity for the δ-opioid receptor and the κ-opioid receptor. In line with these findings, experiments involving intravenous self-administration in rats have shown that bucinnazine possesses a notable reinforcing effect, with tolerance and dependence developing rapidly. Moreover, administering the morphine antagonist naloxone effectively reverses the effects of bucinnazine. It triggers withdrawal symptoms in rats treated with the drug, further supporting the mechanism of analgesia mediated by selective agonist activity at μ-opioid receptors.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 17719-89-0 17730-82-4 (HCl) |
|---|---|
| PubChem CID | 6005081 |
| ChemSpider | 4777510 |
| UNII | J735KL8O54 |
| Chemical and physical data | |
| Formula | C17H24N2O |
| Molar mass | 272.392 g·mol−1 |
Derivatives
2-methyl-AP-237 emerged on the underground market as a designer opioid and was initially detected by a police forensic laboratory in Slovenia in March 2019.
FAQ
- What is Bucinnazine?
- Bucinnazine, or AP-237, is an opioid analgesic drug used to manage pain, particularly in cancer patients. It has been utilized for this purpose in China since 1986.
- How does Bucinnazine compare to other opioids regarding potency and therapeutic index?
- Bucinnazine is one of the most potent compounds among a group of piperazine-amides. It exhibits analgesic potency similar to morphine but maintains a higher therapeutic index.
- Was Bucinnazine initially considered a non-narcotic analgesic?
- Yes, Bucinnazine was initially described as a non-narcotic analgesic. However, subsequent research revealed its potent activity at the μ-opioid receptor (MOR).
- What is the mechanism of action of Bucinnazine as an analgesic?
- Bucinnazine and similar acyl piperazines act as potent and selective agonists of the μ-opioid receptor (MOR). They have relatively low affinity for the δ-opioid receptor and the κ-opioid receptor, which suggests their mechanism of analgesia is primarily mediated via selective agonist activity at μ-opioid receptors.
- Is Bucinnazine associated with tolerance and dependence?
- Yes, experiments have shown that Bucinnazine has a marked reinforcing effect, with tolerance and dependence developing quickly in animal studies.
- Is Bucinnazine available for medical use outside of China?
- As of my last knowledge update in September 2021, Bucinnazine had limited use outside of China and was not widely approved for medical purposes. It’s essential to check its current regulatory status in specific regions.
- Are there potential side effects or risks associated with Bucinnazine use?
- The safety and risks of using Bucinnazine, particularly for extended periods, must be well-documented. Users should exercise caution and consult healthcare professionals when considering its use.
- Where can I find more information about Bucinnazine?
- For more comprehensive information about Bucinnazine, consider referring to scientific literature, research studies, and reliable sources. Stay updated on developments regarding its regulatory status and potential medical applications.
References
- “2-Methyl-AP-237.” Published by the Office of Diversion Control, U.S. Drug Enforcement Administration.
- Qing T, Zhi-Ji C, Wei-Ping W (1986). “Experimental Study on the Dependence-Producing Properties of Qiang Tong Ding (AP-237).” Published in the Chinese Journal of Clinical Pharmacology.
- Nishimura N, Kiuchi M, Kanetake Y, Takahashi T (June 1970). “[Clinical evaluation of a new analgesic agent, AP-237].” Published in Masui: The Japanese Journal of Anesthesiology.
- Carrano RA, Kimura KK, McCurdy DH (January 1975). “Analgesic and tolerance studies with AP-237, a new analgesic.” Published in Archives Internationales de Pharmacodynamie et de Therapie.
- Carrano RA, Kimura KK, Landes RC, McCurdy DH (January 1975). “General pharmacology of a new analgesic – AP-237.” Published in Archives Internationales de Pharmacodynamie et de Therapie.
- Barlocco D, Cignarella G, Greco G, Novellino E (October 1993). “Computer-aided structure-affinity relationships in a set of piperazine and 3,8-diazabicyclo[3.2.1]octane derivatives binding to the mu-opioid receptor.” Published in the Journal of Computer-Aided Molecular Design.
- “Analytical Report 2-Methyl-AP-237.” Published by the National Forensic Laboratory in Ljubljana, Slovenia, on March 19, 2019.
- Fogarty MF, Vandeputte MM, Krotulski AJ, Papsun D, Walton SE, Stove CP, Logan BK (June 2022). “Toxicological and pharmacological characterization of novel cinnamylpiperazine synthetic opioids in humans and in vitro, including 2-methyl AP-237 and AP-238.” Published in the Archives of Toxicology.
- Giorgetti A, Brunetti P, Pelotti S, Auwärter V (October 2022). “Detection of AP-237 and synthetic cannabinoids on an infused letter sent to a German prisoner.” Published in Drug Testing and Analysis.