The proliferation of online vendors offering α-PHiP, a designer drug classified as a research chemical, raises significant concerns within the scientific and ethical communities. While these sellers advertise α-PHiP for sale, promising convenience and accessibility, it is crucial to critically evaluate this practice’s implications.
Firstly, the legitimacy and credibility of α-PHiP research chemical sellers are often questionable. Many vendors operate in a legally gray area, exploiting regulatory loopholes to market and sell substances without proper scientific evaluation. This raises concerns about their products’ quality, purity, and safety. Researchers and consumers cannot be assured that they purchase a reliable compound, potentially jeopardizing experimental outcomes or personal well-being.
Moreover, the online availability of α-PHiP fosters a problematic atmosphere surrounding research chemicals. The term “research chemical” is often used as a euphemism for substances with psychoactive properties without rigorous testing or approval for human consumption. Such unregulated access to potentially harmful compounds undermines scientific research principles, emphasizing safety, transparency, and ethical conduct.
Additionally, the marketing tactics employed by these sellers, including flashy websites and alluring promotions, contribute to the normalization of experimental drug use. This normalization can adversely affect public health and safety, as it encourages experimentation without a clear understanding of the risks involved.
Furthermore, the lack of oversight in the sale of α-PHiP research chemicals allows these substances to evade legal restrictions. This poses challenges for law enforcement and perpetuates the cycle of designer drugs entering the market as soon as one is banned, making it difficult to control their distribution.
Contents
- 1 Summary
- 2 Legal status
- 3 FAQ
- 3.1 1. What is α-PHiP?
- 3.2 2. How does α-PHiP differ from other stimulants?
- 3.3 3. What is the in vitro potency of α-PHiP?
- 3.4 4. Is α-PHiP considered a designer drug?
- 3.5 5. Where can α-PHiP be purchased?
- 3.6 6. What are the risks associated with α-PHiP use?
- 3.7 7. Is α-PHiP legal everywhere?
- 3.8 8. Can α-PHiP be detected in drug tests?
- 3.9 9. Is α-PHiP subject to regulation or control by authorities?
- 4 References
Summary
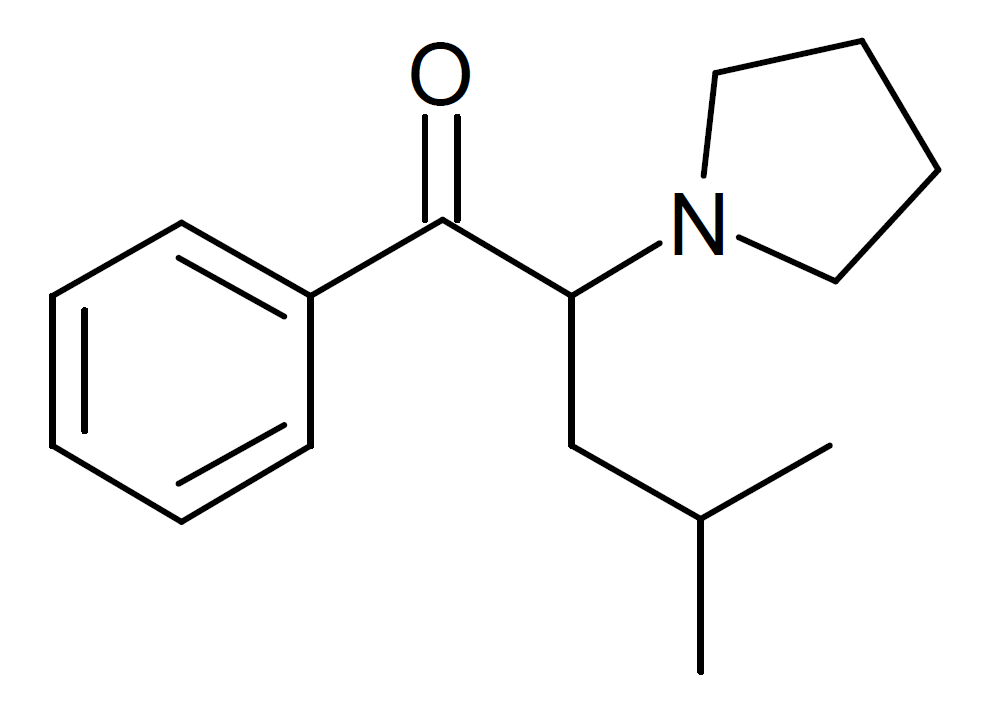
α-PHiP, also called α-PiHP, is a stimulant in the cathinone class of drugs. This compound has gained popularity as a designer drug and has been made available online. Its unique structural characteristic involves a positional isomer of pyrovalerone, where the methyl group has been shifted from the 4-position of the aromatic ring to the 4-position of the acyl chain.
A significant study by Meltzer et al. at Organix in 2006 explored pyrrolidinyl cathinone derivatives. Among the various alpha-substituted derivatives tested, the alpha-isobutyl derivative of pyrovalerone, known as O-2494, exhibited the highest potency in inhibiting the dopamine transporter in vitro.
It’s worth noting that although O-2494 demonstrated this remarkable potency, it wasn’t until a decade later, in July 2016, that α-PHiP was initially identified as a designer drug. This revelation came to light when a forensic laboratory in Slovenia reported its existence to the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA).
This information underscores the evolving landscape of designer drugs and the need for continued vigilance in monitoring and regulating such substances due to their potential risks and implications for public health and safety.
| Identifiers | |
|---|---|
| show IUPAC name | |
| CAS Number | 2181620-71-1 |
| PubChem CID | 59809191 |
| ChemSpider | 68006957 |
| UNII | 9AF2IHV9HK |
| Chemical and physical data | |
| Formula | C16H23NO |
| Molar mass | 245.366 g·mol−1 |
Legal status
| Legal status | BR: Class F2 (Prohibited psychotropics) CA: Schedule I DE: Anlage II (Authorized trade only, not prescriptible)UK: Class B US: Schedule I (positional isomer of a-PHP) |
|---|
FAQ
1. What is α-PHiP?
- α-PHiP, also known as α-PiHP, is a stimulant drug in the cathinone class. It has gained popularity as a designer drug and is available online.
2. How does α-PHiP differ from other stimulants?
- α-PHiP is structurally distinct from other stimulants, such as pyrovalerone, due to a unique positional isomerism where the methyl group is shifted from the 4-position of the aromatic ring to the 4-position of the acyl chain.
3. What is the in vitro potency of α-PHiP?
- In vitro studies have shown that α-PHiP, specifically its alpha-isobutyl derivative O-2494, exhibits high potency as a dopamine transporter inhibitor among alpha-substituted derivatives.
4. Is α-PHiP considered a designer drug?
- Yes, α-PHiP is categorized as a designer drug. It has been synthesized and marketed to mimic the effects of controlled substances while attempting to evade legal restrictions.
5. Where can α-PHiP be purchased?
- α-PHiP has been sold online through various vendors and websites. However, it’s essential to be aware of the legal status of this substance in your region and to exercise caution when considering its purchase.
6. What are the risks associated with α-PHiP use?
- The use of α-PHiP carries potential health risks, including cardiovascular effects, mental health implications, and unknown long-term consequences. The lack of regulation and quality control in producing designer drugs makes them particularly risky.
7. Is α-PHiP legal everywhere?
- The legality of α-PHiP varies by country and jurisdiction. Researching and understanding the specific laws and regulations concerning this substance in your area is essential.
8. Can α-PHiP be detected in drug tests?
- While α-PHiP may not be specifically tested for in standard drug tests, it may produce a positive result for amphetamines due to its stimulant properties and structural similarity to certain controlled substances.
- The regulatory status of α-PHiP differs worldwide. Some regions have taken measures to control or ban it, while others may not have specific regulations. Staying informed about local drug laws is crucial.
References
- Anvisa (2023-07-24). “RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” [Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- Meltzer PC, Butler D, Deschamps JR, Madras BK (February 2006). “1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors”. Journal of Medicinal Chemistry. 49 (4): 1420–32. doi:10.1021/jm050797a. PMC 2602954. PMID 16480278.
- “Analytic Report alpha-PHiP (C16H23NO) 4-methyl-1-phenyl-2-(pyrrolidin-1-yl)pentan-1-one” (PDF). Slovenia: Nacionalni forenzični laboratorij. July 2016. Archived (PDF) from the original on 2020-10-03. Retrieved 2019-10-24.
- “Europol 2016 Annual Report on the implementation of Council Decision 2005/387/JHA” (PDF). EMCDDA–Europol Joint Publication. Archived (PDF) from the original on 2021-01-26. Retrieved 2019-10-24.
- Liu C, Jia W, Li T, Hua Z, Qian Z (August 2017). “Identification and analytical characterization of nine synthetic cathinone derivatives N-ethylhexedrone, 4-Cl-pentedrone, 4-Cl-α-EAPP, propylone, N-ethylnorpentylone, 6-MeO-bk-MDMA, α-PiHP, 4-Cl-α-PHP, and 4-F-α-PHP”. Drug Testing and Analysis. 9 (8): 1162–1171. doi:10.1002/dta.2136. PMID 27863142.