The prevalence of Benzo Fury (6-APB) research chemical sellers online has given rise to substantial concerns within the scientific community and among those interested in acquiring designer drugs for research purposes. While the accessibility of these compounds can offer valuable opportunities for advancing scientific knowledge, the lack of regulation and oversight in this emerging market poses significant risks.
One of the primary issues revolves around the ease with which individuals can buy Benzo Fury and similar research chemicals online. Numerous online vendors claim these substances for sale, often with minimal verification of the buyer’s credentials or the intended research objectives. This lack of scrutiny raises the potential for these substances to fall into the hands of individuals with no genuine research intent, thus increasing the risk of misuse and harm.
Moreover, the quality and purity of Benzo Fury products from different online sellers can vary considerably. With standardized quality control measures, researchers may encounter consistent and reliable results in their experiments, undermining the integrity of the scientific process and potentially leading to erroneous conclusions.
The legality surrounding Benzo Fury and similar research chemicals is another substantial concern. Regulatory frameworks for these substances often need more clarity, and what is legal in one jurisdiction may be prohibited in another. This legal ambiguity places researchers at risk of unintentional violations, which could have serious legal consequences.
Additionally, the limited availability of comprehensive information and data on Benzo Fury’s pharmacology and potential risks complicates responsible use in scientific investigations. More research in this area needs to be conducted to make well-informed decisions about the substances they study.
Contents
- 1 Summary
- 2 History and culture
- 3 Chemistry
- 4 Dosage
- 5 Pharmacology
- 6 Pharmacokinetics
- 7 Subjective effects
- 8 Effects physiology
- 9 Mental effects
- 10 Visual effects of 6-APB
- 11 Reagent results
- 12 Toxicity and harm potential
- 13 Tolerance potential
- 14 Dangerous interactions
- 15 Serotonin syndrome risk
- 16 Legal status
- 17 FAQ
- 17.1 1. What is Benzo Fury (6-APB)?
- 17.2 2. What are the effects of Benzo Fury?
- 17.3 3. Is Benzo Fury legal?
- 17.4 4. Is Benzo Fury safe to use?
- 17.5 5. How do I use Benzo Fury safely?
- 17.6 6. Are there any interactions to be aware of when taking Benzo Fury (6-APB)?
- 17.7 7. What precautions should one take when using Benzo Fury (6-APB)?
- 17.8 8. Can Benzo Fury (6-APB) be used for therapeutic purposes?
- 17.9 9. Can Benzo Fury (6-APB) lead to addiction or dependence?
- 18 References
Summary
6-(2-Aminopropyl)benzofuran, also known as 6-APB or “Benzofury,” represents a unique entactogen compound within the benzofuran class. It shares structural similarities with well-known entactogens such as MDA, MDMA, 5-APB, and 5-MAPB.
The synthesis of 6-APB can be traced back to 1993, when David E. Nichols developed it as a potential non-neurotoxic alternative to MDMA. However, its recreational use by humans was not documented until more than a decade later, when it briefly appeared in the rave scene and the global research chemical market. It was marketed alongside other novel benzofuran entactogens under the moniker “Benzofury” before regulations led to its ban.
Subjective experiences associated with 6-APB encompass anxiety reduction, disinhibition, muscle relaxation, and euphoria. Its effects are frequently likened to those of MDA and other entactogenic substances.
Despite its presence in the recreational domain, there is minimal available data regarding the pharmacological properties, metabolism, and toxicity of 6-APB. Additionally, its history of human usage is relatively short. 6-APB has been promoted as a legal, grey-market substitute for MDMA, often distributed by online research chemical vendors. It is crucial for individuals considering its use to adhere to harm reduction practices.
| Identifiers | |
|---|---|
| show IUPAC name | |
| CAS Number | 286834-85-3 286834-84-2 (HCl) |
| PubChem CID | 9794343 |
| ChemSpider | 7970110 |
| UNII | 285VE60914 |
| CompTox Dashboard (EPA) | DTXSID401010105 |
| Chemical and physical data | |
| Formula | C11H13NO |
| Molar mass | 175.231 g·mol−1 |
History and culture
The synthesis of 6-APB was initially documented by a team led by the renowned medicinal chemist and psychedelic researcher David E. Nichols at Purdue University. Their research aimed to explore the interaction between the MDA dioxide ring structure and serotonergic neurons. Additionally, it served as an endeavor to discover an alternative to MDMA, a substance gaining recognition for its potential utility in psychotherapy but also associated with neurotoxic effects.
Although human usage remained undocumented until 2010, it eventually surfaced for sale on the research chemical market. Notably, it gained prominence in the UK’s “legal highs” market, marketed under the “Benzofury.”
On June 10, 2013, 6-APB and several analogs were provisionally classified as Temporary Class Drugs in the UK, in alignment with a recommendation from the ACMD (Advisory Council on the Misuse of Drugs). Subsequently, on November 28, 2013, the ACMD proposed that 6-APB and related benzofurans should be categorized as Class B, Schedule 1 substances. On March 5, 2014, the UK Home Office formally announced that 6-APB would be reclassified as a class B drug, effective from June 10, 2014, alongside numerous other benzofuran entactogens and structurally related compounds.
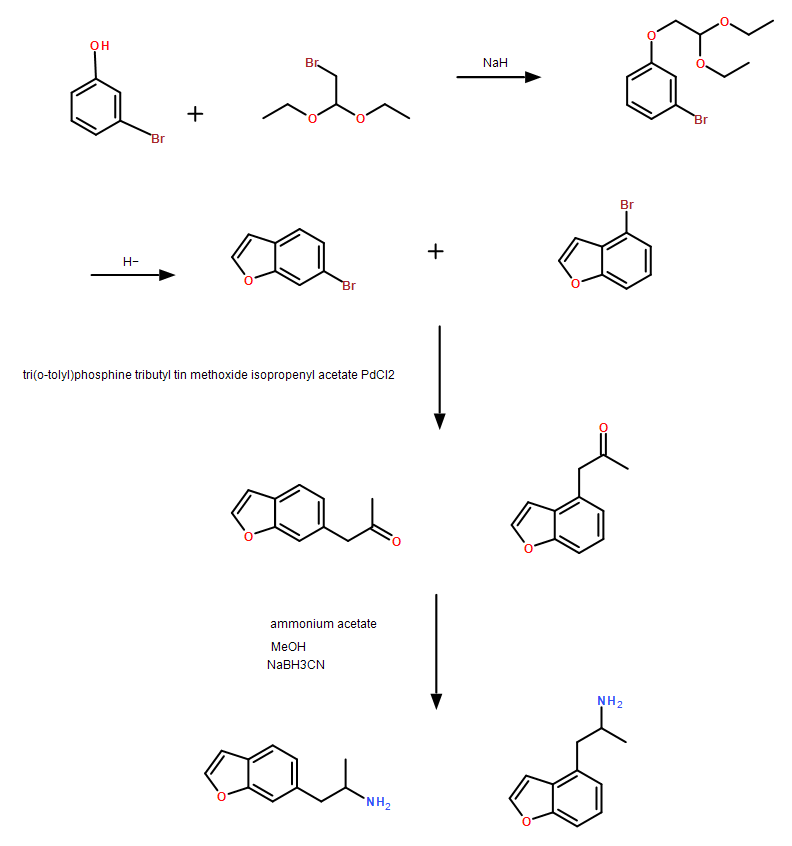
Chemistry
6-APB, also scientifically named 6-(2-aminopropyl)benzofuran, belongs to the synthetic benzofuran class of compounds. Benzofurans are part of the broader amphetamine and phenylethylamine classes of substances. These molecules typically feature a phenethylamine core linked to an amino (NH2) group through an ethyl chain, often with an additional methyl substitution at Rα. It’s worth noting that 6-APB lacks a methyl substitution on RN. Its structure consists of an oxygen-substituted benzofuran ring fused at positions R3 and R4 of the phenyl ring.
6-APB shares this benzofuran ring with related compounds like 5-APB, 5-MAPB, and 6-MAPB.
Since its initial introduction to the market, three distinct batches of 6-APB have circulated. Initially, only the hydrochloride form was available, with a dosage range resembling that of MDA regarding dose-response. However, succinate and fumarate batches subsequently appeared in the market, and they exhibit varying effects by weight and significantly different loose bulk densities.
Dosage
Pharmacology
6-APB, scientifically called 6-(2-aminopropyl)benzofuran, operates as a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI). It exhibits distinct binding affinities, with Ki values of 117 nM for the norepinephrine transporter (NET), 150 nM for the dopamine transporter (DAT), and 2698 nM for the serotonin transporter (SERT). Additionally, 6-APB can act as a releasing agent, promoting the release of these monoamine neurotransmitters.
Remarkably, 6-APB is a potent full agonist of the serotonin 5-HT2B receptor, boasting a Ki value of 3.7 nM. It exhibits a higher affinity for this receptor than any other target site, and it stands apart from MDMA by displaying a 100-fold selectivity for the 5-HT2B receptor over the 5-HT2A and 5-HT2C receptors.
Beyond its interaction with the 5-HT2B receptor, 6-APB also exhibits high-affinity binding to the α2C-adrenergic receptor subtype, with a Ki value of 45 nM, although the clinical significance of this action remains uncertain.[4]
The potent agonism of the 5-HT2B receptor suggests a potential for cardiotoxicity with chronic or long-term use, a concern shared with other 5-HT2B receptor agonists like the withdrawn serotonergic anorectic fenfluramine.
Monoamine neurotransmitters, including serotonin, dopamine, and noradrenaline, play pivotal roles in regulating various brain functions related to pleasure, motivation, reward, attention, and focus. Inhibiting their reuptake or enhancing their release leads to accumulating these neurotransmitters in the synaptic cleft, facilitating their reuse. This results in neuronal activation across multiple brain regions, culminating in stimulating, relaxing, disinhibiting, and euphoric effects.

Pharmacokinetics
While formal pharmacokinetic studies on 6-APB are lacking, some insights can be gleaned from user accounts. These reports indicate a gradual onset of effects within 40 to 120 minutes. The peak effects of the drug typically endure for approximately 7 hours, followed by a comedown phase lasting around 2 hours. Additionally, users may experience after-effects for up to 24 hours following their initial consumption.
Subjective effects
Disclaimer: The effects mentioned below are derived from the Subjective Effect Index (SEI), which relies on anecdotal user reports and the individual assessments of contributors to PsychonautWiki. Therefore, it is essential to approach these findings with a measure of skepticism.
Furthermore, it’s essential to recognize that these effects may manifest inconsistently or predictably. Generally, higher doses are more likely to produce a broader range of products. Conversely, as amounts increase, the likelihood of adverse consequences, including addiction, serious harm, or fatality, also rises.

Effects physiology
Stimulation & Sedation: 6-APB is known for its unique ability to stimulate and relax the user’s physical energy levels simultaneously. It is generally considered less energetically stimulating than MDMA or MDA, often inducing a pronounced “stoning” or “couch-locking” sensation. The style of stimulation it offers is gentler, akin to that experienced with psychedelics like mescaline.
Spontaneous Physical Sensations:
He “body high” induced by 6-APB can be characterized as a moderate to powerful, warm, and euphoric tingling sensation that permeates the entire body. At higher doses, it can become overwhelmingly pleasurable, to the point of immobilizing the user. This sensation remains consistently present, intensifying as the effects set in and reaching its zenith during the peak.
Tactile Enhancement:
Users commonly report heightened tactile sensations, making the sense of touch more vivid and enjoyable.
Bodily Control Enhancement: 6-APB often enhances bodily control, giving users a heightened sense of physical coordination.
Stamina Enhancement:
Users may experience increased stamina, potentially leading to extended physical activities.
Temperature Regulation Suppression: 6-APB can potentially suppress the body’s ability to regulate temperature efficiently. It is essential to exercise caution, as excessive doses can disrupt the body’s internal temperature control mechanisms. Reports suggest that 6-APB’s hyperthermia is somewhat warmer than MDA and MDMA, although similar. This effect can reach dangerous levels, potentially contributing to severe and life-threatening serotonin syndrome.
Vibrating Vision:
At moderate to high doses, individuals may notice rapid, involuntary eye movements, causing temporary blurriness and difficulty focusing. This condition is known as nystagmus.
Abnormal Heartbeat
Increased Heart Rate
Increased Blood Pressure
Increased Perspiration
Dehydration:
Dry mouth and dehydration are common side effects associated with substances in this class. These effects arise from an elevated heart rate and increased metabolic activity. While it is essential to stay hydrated, users should exercise caution to prevent overhydration, as there have been cases of water intoxication due to excessive drinking. Users are advised to monitor their water intake carefully.
Dry Mouth
Difficulty Urinating: Higher doses of 6-APB can lead to temporary difficulty urinating. This effect is harmless and short-lived. It results from 6-APB’s influence on the release of anti-diuretic hormone (ADH), which regulates urination. Relaxation and warmth, such as applying a warm compress to the genital area, can alleviate this effect.
Appetite Suppression
Pupil Dilation
Excessive Yawning
Temporary Erectile Dysfunction
Teeth Grinding: Teeth grinding is more likely to occur at moderate to higher doses, similar to what is experienced with MDMA or MDA.
Seizure:
Although rare, seizures may occur, particularly in individuals predisposed to them. This risk is heightened with potent doses or redosing in physically demanding conditions, such as dehydration, fatigue, malnutrition, or overheating.
Mental effects
The cognitive effects of 6-APB can be categorized into various aspects that become more pronounced with increasing dosage. Generally, the mental state induced by 6-APB is described as moderately mentally stimulating, accompanied by feelings of love, openness, empathy, and a potent sense of contentment and euphoria. It exhibits various typical cognitive effects, including those associated with psychedelics, entactogens, and stimulants. The most notable mental products often include:
- Anxiety Suppression
- Disinhibition
- Delusion
- Cognitive Euphoria: A strong sense of emotional euphoria and happiness, likely stemming from serotonin and dopamine release.
- Empathy, Affection, and Sociability Enhancement: This effect is remarkably consistent, powerful, and therapeutic with 6-APB, surpassing most other substances. It prominently shapes the mental state during a 6-APB experience, emphasizing communication and bonding. However, with repeated use and inadequate spacing, this effect may diminish, making users feel more speedy and scattered, with reduced urges to connect with others.
- Novelty Enhancement
- Creativity Enhancement
- Focus Enhancement: Most effective at low to moderate doses, as higher doses tend to impair concentration.
- Immersion Enhancement
- Motivation Enhancement
- Memory Suppression: Considered mild compared to substances like alcohol and traditional benzodiazepines, resulting in a somewhat “spaced-out” feeling characterized by episodic memory lapses.
- Emotion Enhancement
- Increased Music Appreciation
- Personal Meaning Enhancement
- Increased Sense of Humor
- Laughter Fits
- Compulsive Redosing: Due to its potential for inducing euphoria, 6-APB may lead to compulsive redosing, akin to MDMA or MDA. Given the duration of the experience and reports of an amplified offset (“comedown”) and after-effects, redosing is strongly discouraged. This aspect shares similarities with compounds like DOM and related substances.
- Increased Libido
- Mindfulness
- Thought Acceleration
- Time Distortion: Strong sensations of time compression are familiar with 6-APB, altering the perception of time, although to a lesser extent than typically reported with MDMA.
- Wakefulness: This effect is notably less pronounced at present than with MDMA. Users often report feeling heavily sedated or “floored” compared to typical stimulants.
Visual effects of 6-APB
Visual effects induced by 6-APB are characterized by their selective occurrence and lesser consistency compared to traditional psychedelics. These effects cannot be guaranteed but are more likely to manifest with high doses toward the end of the experience, especially with cannabis use. They are also more probable if the user has prior experience with psychedelics, but can occur in individuals without previous exposure.
Enhancements:
6-APB elicits mild visual enhancements distinct from traditional psychedelics, including:
- Color Enhancement: Colors appear vivid and synthetic throughout the experience.
- Pattern Recognition Enhancement
- Distortions
- Tracers: Tracers are akin to those observed with MDA, featuring longer continuity sections before a slight discontinuity.
- **Symmetrical Texture Repetition
Geometry:
The visual geometry experienced with 6-APB is more reminiscent of mescaline than LSD or psilocin. These geometric patterns are primarily intricate, abstract, organic in style, well-structured, dimly lit, predominantly monotone with shades of blues and greys, glossy in shading, sharp-edged, small in size, fast-moving, smooth in motion, featuring both round and angular corners, non-immersive, and consistent in intensity. They are more likely to reach level 8A visual geometry at higher doses rather than level 8B.
Hallucinatory States:
6-APB can induce a unique range of low and high-level hallucinatory states at high to heavy doses, which are less consistent and reproducible than many other commonly used psychedelics. These effects are more prevalent during the experience’s offset and may include:
- Transformations
- External Hallucination: This effect is similar to experiences with deliriants but is inconsistent and usually occurs at high doses. It includes autonomous entities, settings, sceneries, landscapes, perspective hallucinations, scenarios, and plots. These hallucinations are characterized by their delirious believability, controllable autonomy, and solid style. They often revolve around memory replays and semi-realistic or expected events, such as seeing people holding objects or performing actions that one would expect in real life before these images dissolve under scrutiny.
- Internal Hallucination: These internal hallucinations are typically spontaneous breakthroughs at extremely high doses. They feature delirious believability, interactive style, novel content, controllable autonomy, and solid appearance. They often manifest as hypnagogic scenarios experienced as the user drifts off to sleep after using the substance. These scenarios typically involve memory replays from the previous hours and are short, fleeting, frequent, and entirely convincing. They often revolve around conversations with people who were present or nonsensical and bizarre plots.
- Peripheral Information Misinterpretation
Reagent results
Exposing compounds to the reagents gives a colour change which is indicative of the compound under test.
| Compound | Marquis | Mecke | Mandelin | Liebermann | Froehde | Gallic | Ehrlich | Hofmann | Simon’s | Folin |
|---|---|---|---|---|---|---|---|---|---|---|
| 6-APB | Purple | Purple – black | Purple – black | Black | Purple | Brown | Orange | Light orange | No reaction | Light orange |
| 6-APB succinate | Purple | Purple – black | Purple – black | Black | Purple | Brown | Faint orange | No reaction | No reaction | Light orange |
Toxicity and harm potential
Due to its relatively brief history of human use, the exact toxicity and potential for harm associated with 6-APB remain uncertain. Given its structural similarity to MDMA, there is a likelihood that repeated or high doses of 6-APB may carry neurotoxic and cardiotoxic risks in some capacity. The precise toxic dosage remains unknown, underscoring the importance of practicing harm reduction when using this substance.
Short-term physical health risks associated with 6-APB use include dehydration, insomnia, and hyperthermia (overheating). Continuous activity without adequate rest or hydration can elevate body temperature to hazardous levels. Additionally, excessive sweating, coupled with the stimulating and euphoric effects of the substance, may lead users to underestimate their energy expenditure. The use of diuretics like alcohol can exacerbate these risks.
Although it has not been formally studied, small ambient temperature fluctuations may significantly impact 6-APB-induced serotonergic neurotoxicity, similar to MDMA. The neurotoxicity of 6-APB remains a topic of ongoing debate. It was initially designed to be less neurotoxic than MDA or MDMA by avoiding the production of inevitable metabolic byproducts associated with their toxicity, specifically alpha-methyl-dopamine. While it is likely safe when used responsibly, there is a possibility that repeated or high doses of 6-APB could lead to neurotoxicity, resulting in cognitive, emotional, and psychomotor function deficits.
Like MDMA, long-term heavy usage of 6-APB (such as regular daily or weekly use) is likely cardiotoxic and may increase the risk of valvulopathy (heart valve issues) due to its significant affinity for the 5-HT2B receptor.
- It is crucial to note that 6-APB should not be insufflated, as its fine powder can lead to severe coughing and reduced subjective effects. Oral consumption is considered a safer route of administration.
Tolerance potential
Like other stimulants, chronic use of 6-APB carries a moderate risk of addiction and a high potential for abuse, potentially leading to psychological dependence in some individuals. Those addicted may experience cravings and withdrawal symptoms if they abruptly cease their use.
Due to its potent serotonin-releasing properties, tolerance to 6-APB develops rapidly with prolonged and repeated consumption. This tolerance can reach a point where the substance no longer produces positive effects but instead induces an uncomfortable state of anxious and dysphoric stimulation. Consequently, users may need to administer progressively larger doses to achieve the desired results. It typically takes around 3-4 weeks for tolerance to decrease by half and 6-8 weeks to return to baseline levels without further consumption.
Furthermore, 6-APB exhibits cross-tolerance with all dopaminergic stimulants, meaning that its use can reduce the effectiveness of other stimulant substances.
Dangerous interactions
Warning: Combining certain substances can pose significant risks, including potential harm or life-threatening situations. It is crucial to conduct thorough independent research (e.g., using search engines like Google or DuckDuckGo or consulting scientific publications on platforms like PubMed) to ensure the safety of combining two or more substances. The following list highlights known dangerous interactions, but it may not cover all potential risks:
- 25x-NBOMe & 25x-NBOH – These compounds are highly stimulating and physically taxing. Combining them with 6-APB should be strictly avoided due to the potential for excessive stimulation and strain on the heart. Possible outcomes include elevated blood pressure, vasoconstriction, panic attacks, thought loops, seizures, and, in extreme cases, heart failure.
- Alcohol – Mixing alcohol with stimulants can be hazardous because stimulants can mask the depressant effects of alcohol. This can lead to unintentional over-intoxication. Once the stimulant’s effects wear off, the depressant effects of alcohol may become dominant, potentially resulting in blackouts and severe respiratory depression. If combining these substances, it is essential to limit alcohol consumption to a specific amount per hour.
- DXM – Avoid combining DXM with 6-APB due to DXM’s inhibitory effects on serotonin and norepinephrine reuptake. This combination increases the risk of panic attacks, hypertensive crisis, or serotonin syndrome when used with serotonin releasers like MDMA, methylone, or mephedrone. Monitor blood pressure carefully and avoid strenuous physical activity.
- MDMA – Combining MDMA with other stimulants can amplify any neurotoxic effects of MDMA. It may also lead to increased blood pressure and strain on the heart (cardiotoxicity).
- MXE – Some reports suggest mixing MXE with 6-APB can significantly raise blood pressure and increase the risk of mania and psychosis.
- Dissociatives – Both 6-APB and dissociatives carry a risk of inducing delusions, mania, and psychosis. Combining them may increase these risks.
- Stimulants – Combining 6-APB with other stimulants like cocaine can elevate heart rate and blood pressure to dangerous levels. This combination should be approached with caution.
- Tramadol – Tramadol is known to lower the seizure threshold, and combining it with stimulants may increase the risk of seizures.
- MAOIs – Combining 6-APB with MAOIs may lead to dangerously elevated levels of neurotransmitters like dopamine, potentially resulting in fatal consequences. Examples of MAOIs include Syrian rue, banisteriopsis caapi, and certain antidepressants.
It is imperative to exercise caution and prioritize safety when considering the combination of any substances, as adverse effects and risks can vary widely depending on factors such as dosage, individual sensitivity, and overall health.
Serotonin syndrome risk
Combining 6-APB with certain substances can lead to dangerously elevated serotonin levels, potentially resulting in serotonin syndrome. This severe medical condition requires immediate attention and can be fatal if left untreated. The following substances are known to increase the risk of serotonin syndrome when used in conjunction with 6-APB:
- MAOIs – Examples include banisteriopsis caapi, Syrian rue, phenelzine, selegiline, and moclobemide.
- Serotonin releasers release serotonin, including MDMA, 4-FA, methamphetamine, methylone, and αMT.
- SSRIs – Selective serotonin reuptake inhibitors like citalopram and sertraline.
- SNRIs – Serotonin-norepinephrine reuptake inhibitors such as tramadol and venlafaxine.
- 5-HTP – A precursor to serotonin.
It is essential to exercise extreme caution and avoid combining 6-APB with any of these substances to prevent the potentially life-threatening complications associated with serotonin syndrome. If you suspect serotonin syndrome or experience symptoms such as agitation, confusion, rapid heart rate, high blood pressure, dilated pupils, muscle rigidity, heavy sweating, diarrhea, or tremors, seek immediate medical assistance.
Legal status
6-APB’s legal status varies from country to country:
Australia and New Zealand have a “substantially similar” catch-all clause in their drug laws. This includes 6-APB due to its structural similarity to the class A drug MDA, potentially classifying it as a controlled substance analog.
Canada:
6-APB is classified as Schedule III in Canada, as it is considered an analog of MDA. This classification was updated due to changes in the Controlled Drugs and Substances Act (CDSA) resulting from the Safe Streets Act.
Czech Republic:
6-APB is categorized as a Schedule I substance, allowing limited use for research and restricted therapeutic purposes.
Germany:
As of July 17, 2013, 6-APB is controlled under Anlage II BtMG (Narcotics Act, Schedule II) in Germany. Manufacturing, possessing, importing, exporting, buying, selling, procuring, or dispensing it without a license is illegal.
Italy:
6-APB is illegal in Italy.
Japan:
6-APB has been a controlled substance in Japan since December 17, 2012.
Luxembourg:
6-APB is not listed as a prohibited substance in Luxembourg, making it a legal sense.
Sweden:
Sweden has classified 6-APB as a “health hazard” since 2009, making it illegal.
Switzerland:
6-APB is a controlled substance named explicitly under Verzeichnis E in Switzerland.
Turkey:
6-APB is classified as a drug and is illegal to possess, produce supply, or import in Turkey.
United Kingdom:
In the U.K., 6-APB and some analogs were classified as Temporary Class Drugs on June 10, 2013, following an ACMD recommendation. On March 5, 2014, the U.K. Home Office announced that 6-APB would be reclassified as a class B drug starting from June 10, 2014, along with other benzofuran entactogens and related medications.
United States:
6-APB is unscheduled in the United States, but it is not currently approved by the Food and Drug Administration (FDA) for human consumption.
6-APB is illegal in Germany since the 17th of July, 2013, when it was added to Anlage II of the Betäubungsmittelgesetz.
| Legal status | |
|---|---|
| Legal status | CA: Schedule III DE: Anlage II (Authorized trade only, not prescriptible) UK: Class B |
FAQ
1. What is Benzo Fury (6-APB)?
Benzo Fury, a synthetic research chemical and entactogen, is a 6-APB or 6-(2-aminopropyl)benzofuran. It belongs to the benzofuran class of substances structurally related to compounds like MDMA (Ecstasy).
2. What are the effects of Benzo Fury?
The effects of Benzo Fury can include anxiety suppression, euphoria, disinhibition, increased sociability, enhanced tactile sensations, and stimulation. It’s often compared to the effects of MDMA or MDA.
3. Is Benzo Fury legal?
The legal status of Benzo Fury varies by country. In some places, it is controlled or banned due to its chemical structure or similarity to controlled substances. It’s essential to check your local laws and regulations before obtaining or using it.
4. Is Benzo Fury safe to use?
The safety of using Benzo Fury needs to be well-documented, and its long-term effects are unknown. As with any research chemical, there are potential risks associated with use, including health hazards and unknown purity. It’s crucial to exercise caution and practice harm reduction if considering its use.
5. How do I use Benzo Fury safely?
Some potential risks associated with Benzo Fury use include increased heart rate, elevated blood pressure, dehydration, anxiety, and in some cases, more severe adverse reactions. Overdosing can lead to serious health issues and potentially life-threatening situations.
6. Are there any interactions to be aware of when taking Benzo Fury (6-APB)?
Benzo Fury may interact with certain medications, substances, or pre-existing health conditions. It is crucial to consult with a healthcare professional before using Benzo Fury, especially if one is taking any medications or has any underlying health issues.
7. What precautions should one take when using Benzo Fury (6-APB)?
Precautions include testing the substance for purity and dosage accuracy, using the substance in a safe and controlled environment, having a trusted and sober individual present, and avoiding mixing it with other substances.
8. Can Benzo Fury (6-APB) be used for therapeutic purposes?
While there is some interest in the potential therapeutic applications of Benzo Fury, more research is needed to fully understand its therapeutic potential and any associated risks.
9. Can Benzo Fury (6-APB) lead to addiction or dependence?
While Benzo Fury is not believed to be physically addictive, psychological dependence and compulsive use can occur in some individuals. It is essential to use it responsibly and with caution.
References
- Iversen L, Gibbons S, Treble R, Setola V, Huang XP, Roth BL (January 2013). “Neurochemical profiles of some novel psychoactive substances”. European Journal of Pharmacology. 700 (1–3): 147–51. doi:10.1016/j.ejphar.2012.12.006. PMC 3582025. PMID 23261499.
- Rickli A, Kopf S, Hoener MC, Liechti ME (July 2015). “Pharmacological profile of novel psychoactive benzofurans”. British Journal of Pharmacology. 172 (13): 3412–25. doi:10.1111/bph.13128. PMC 4500375. PMID 25765500.
- Jump up to:a b Canal CE, Murnane KS (January 2017). “2C receptor and the non-addictive nature of classic hallucinogens”. Journal of Psychopharmacology. 31 (1): 127–143. doi:10.1177/0269881116677104. PMC 5445387. PMID 27903793.
- Jump up to:a b US patent 7045545, Briner K, Burkhart JP, Burkholder TP, Fisher MJ, Gritton WH, Kohlman DT, Liang SX, Miller SC, Mullaney JT, Xu YC, Xu Y, “Aminoalkylbenzofurans as serotonin (5-HT(2c)) agonists”, published 19 January 2000, issued 16 May 2006, assigned to Eli Lilly Co.
- Jump up to:a b c Browne J (4 June 2013). “Temporary class drug order on benzofury and NBOMe compounds – letter from ACMD”. Advisory Council on the Misuse of Drugs. GOV.UK.
- Shaun L. Greene (2013). Novel Psychoactive Substances: Classification, Pharmacology and Toxicology Chapter 16 – Benzofurans and Benzodifurans. Boston: Academic Press. pp. 383–392. doi:10.1016/B978-0-12-415816-0.00016-X. ISBN 978-0-12-415816-0.
- Nugteren-van Lonkhuyzen JJ, van Riel AJ, Brunt TM, Hondebrink L (December 2015). “Pharmacokinetics, pharmacodynamics and toxicology of new psychoactive substances (NPS): 2C-B, 4-fluoroamphetamine and benzofurans”. Drug and Alcohol Dependence. 157: 18–27. doi:10.1016/j.drugalcdep.2015.10.011. PMID 26530501.
- Chan WL, Wood DM, Hudson S, Dargan PI (September 2013). “Acute psychosis associated with recreational use of benzofuran 6-(2-aminopropyl)benzofuran (6-APB) and cannabis”. Journal of Medical Toxicology. 9 (3): 278–81. doi:10.1007/s13181-013-0306-y. PMC 3770991. PMID 23733714.
- “Results”. PRO Test. Retrieved 2021-06-01.
- Jump up to:a b Casale JF, Hays PA (January 2012). “The Characterization of 6-(2-Aminopropyl)benzofuran and Differentiation from its 4-, 5-, and 7-Positional Analogues” (PDF). Microgram Journal. 9 (2): 61–74. Archived from the original (PDF) on 2016-11-05. Retrieved 2016-06-08.
- “Controlled Drugs and Substances Act : Definitions and Interpretations”. isomerdesign.com. Retrieved 2019-11-11.
- Hudson AL, Lalies MD, Baker GB, Wells K, Aitchison KJ (2014-04-16). “Ecstasy, legal highs and designer drug use: A Canadian perspective”. Drug Science, Policy and Law. 1: 2050324513509190. doi:10.1177/2050324513509190. ISSN 2050-3245. S2CID 159675835.
- “21 CFR — SCHEDULES OF CONTROLLED SUBSTANCES §1308.11 Schedule I.” Archived from the original on 2009-08-27. Retrieved 2018-04-10.
- Casale JF, Hays PA (January 2011). “The characterization of 5-and 6-(2-aminopropyl)-2, 3-dihydrobenzofuran”. Microgram J. 8 (2): 62–74.
- “Nuove tabelle delle sostanze stupefacenti e psicotrope (DPR 309/90)” (PDF) (in Italian). Ministero della Salute. Archived from the original (PDF) on 2016-02-06. Retrieved 2016-05-31.
- “Loi du 19 février 1973 concernant la vente de substances médicamenteuses et la lutte contre la toxicomanie. – Legilux”. legilux.public.lu. Retrieved 2021-06-01.
- “Misuse of Drugs Act 1975”. New Zealand Legislation. 7 November 2015.
- “Förordning (1999:58) om förbud mot vissa hälsofarliga varor” (in Swedish). Lagbevakning med Notisum och Rättsnätet. 24 November 2009. Archived from the original on 4 October 2013. Retrieved 15 September 2013.
- Browne J (4 June 2013). “‘NBOMe’ and ‘Benzofury’ banned”. Home Office. GOV.UK.
- UK Home Office (28 April 2014). “The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014”. The National Archives.