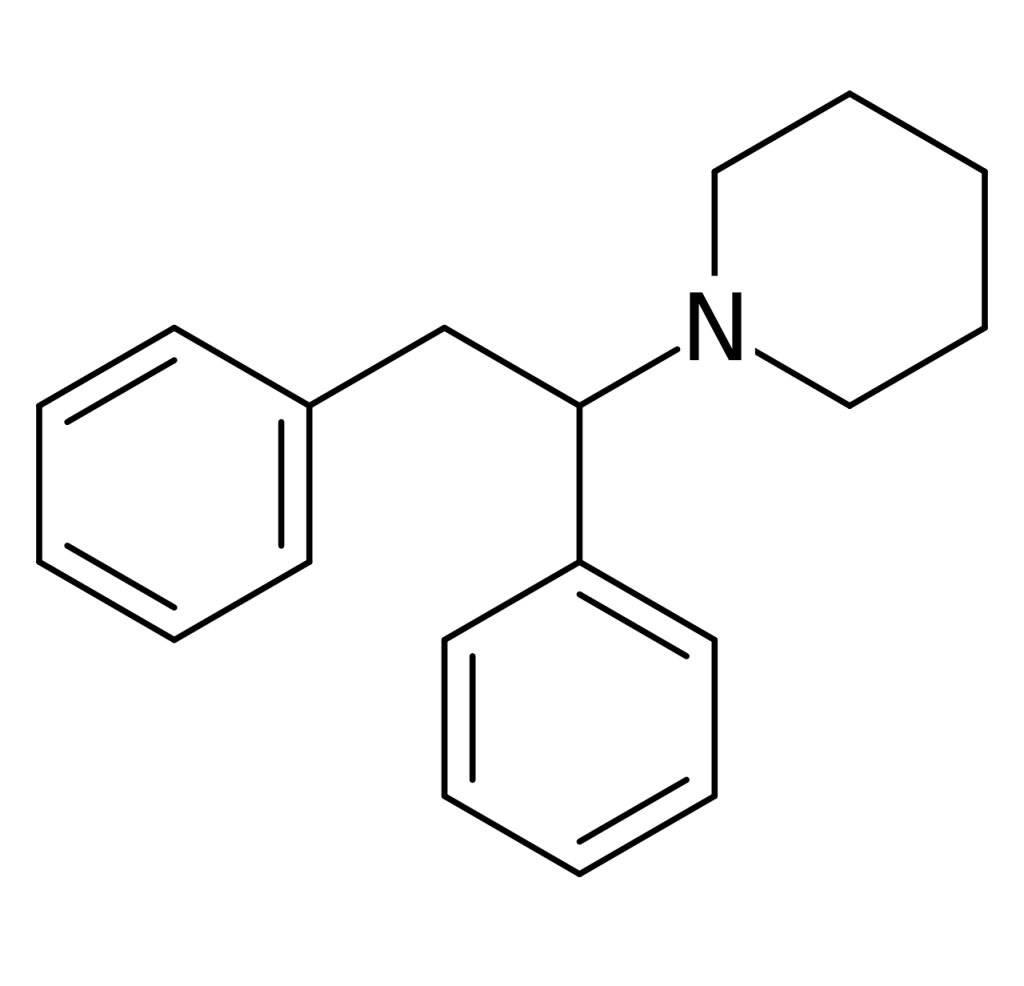
Summary
Diphenidine, also known as 1,2-DEP, DPD, or DND, is a dissociative anesthetic that has been distributed as a designer drug. The synthesis of piperidine dates back to 1924 when a method employing a Bruylants reaction was first reported, a process that would later be utilized to discover phencyclidine in 1956. Following the 2013 ban on arylcyclohexylamines in the UK, diphenidine and its close relative, methoxphenidine, emerged on the grey market.
Reports from users have described high doses of diphenidine as capable of inducing “bizarre somatosensory phenomena and transient anterograde amnesia.” Diphenidine, alongside related diarylethylamines, has been the subject of in vitro research as a potential treatment for neurotoxic injury and has been identified as an antagonist of the NMDA receptor. In the context of antitussive properties, diphenidine exhibits greater potency than codeine phosphate in dogs.
Electrophysiological studies have revealed that diphenidine, similar to ketamine, significantly reduces the amplitude of NMDA-mediated fEPSPs, albeit with a slower onset of antagonism. The two enantiomers of diphenidine exhibit substantial differences in their ability to block the NMDA receptor, with the (S)-enantiomer displaying affinity forty times higher than the (R)-enantiomer. Despite vendors claiming that diphenidine “acts on dopamine transport” since its introduction in 2013, data regarding its impact on the dopamine transporter was not published until 2016. While the NMDA receptor remains the primary target of diphenidine, it also displays submicromolar affinity for the σ1 receptor, σ2 receptor, and dopamine transporter.
From 2014 onward, there have been documented instances of piperidine being combined with other research chemicals, particularly synthetic cannabinoids and stimulants, in Japanese herbal incense blends. Notably, the first reported seizure involved a Japanese product named “fragrance powder” containing diphenidine and benzylpiperazine. In the Shizuoka Prefecture, a herbal incense product labeled “Aladdin Spacial [sic] Edition” was found to have diphenidine and 5F-AB-PINACA at concentrations of 289 mg/g and 55.5 mg/g, respectively. Tragically, a product known as “Herbal Incense. The Super Lemon,” containing AB-CHMINACA, 5F-AMB, and piperidine, was linked to a fatal poisoning incident. Most recently, a deadly ingestion case in Japan involved piperidine in conjunction with three substituted cathinone, three benzodiazepines, and alcohol, all consumed in “bath salt” and “liquid aroma” products.
In Canada, the legal classification of MT-45 and its analogs as Schedule I controlled substances encompassed DPD within its structural group. Unauthorized possession can result in a maximum prison sentence of seven years. Moreover, in May 2016, Health Canada amended the Food and Drug Regulations to explicitly categorize DPD as a restricted drug, allowing only law enforcement agencies, individuals with an exemption permit, or authorized institutions to possess it.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 36794-52-2 |
|---|---|
| PubChem CID | 206666 |
| ChemSpider | 179031 |
| UNII | H8Q4VPL82Y |
| CompTox Dashboard (EPA) | DTXSID50724547 |
| Chemical and physical data | |
| Formula | C19H23N |
| Molar mass | 265.400 g·mol−1 |
FAQ
1. What is Diphenidine?
Diphenidine is a dissociative anesthetic drug that has been sold as a designer drug. It was first synthesized in 1924 and is classified as a diarylethylamine.
2. How does Diphenidine work?
Diphenidine is believed to function as an NMDA receptor antagonist. It produces sedative, hallucinogenic, and anesthetic effects, but it is less potent than ketamine.
3. Are there any reported effects of Diphenidine use?
Reports from users suggest that high doses of Diphenidine can lead to “bizarre somatosensory phenomena and transient anterograde amnesia.”
4. What research has been conducted on Diphenidine?
Diphenidine and related diarylethylamines have been studied in vitro as potential treatments for neurotoxic injury. Electrophysiological studies have demonstrated its impact on NMDA receptors.
5. Is Diphenidine commonly mixed with other substances?
Yes, there have been documented cases of Diphenidine being combined with other research chemicals, particularly synthetic cannabinoids and stimulants, in herbal incense blends.
6. Has Diphenidine been involved in any adverse incidents?
Yes, there have been incidents of adverse effects and fatalities associated with Diphenidine, especially when combined with other substances.
7. Is Diphenidine legally regulated?
Regulations vary by country. In Canada, Diphenidine is classified as a Schedule I controlled substance, and Health Canada has amended rules to classify it as a restricted drug. It is essential to check local laws and regulations in your area.
8. Is Diphenidine safe for recreational use?
The safety of Diphenidine for recreational use is not well-established, and its use can be associated with significant risks. Using any psychoactive substance comes with potential dangers, and it is crucial to exercise caution and stay informed about local laws and regulations.
9. How should I use Diphenidine if it is legal in my area?
If you plan to use Diphenidine where it is legal, it is advisable to start with a low dose and be aware of its potential effects and side effects. Always follow harm reduction principles and avoid combining it with other substances.
10. Where can I find more information about Diphenidine?
For up-to-date and accurate information on Diphenidine, refer to reputable sources such as government health agencies, scientific literature, and drug education websites. Always prioritize your safety and well-being when considering the use of any psychoactive substance.
References
- Anvisa (2023-07-24): In a resolution by the Collegiate Board, denoted as Resolution No. 804, a comprehensive list of narcotic, psychotropic, precursor, and other substances under special control was introduced. This resolution was published in Brazilian Portuguese in the Diário Oficial da União on 2023-07-25, and the archived version is accessible until 2023-08-27, with retrieval documented on the same date.
- Morris H, Wallach J (July–August 2014): The non-medical utilization of dissociative drugs, spanning from PCP to MXE, was extensively reviewed in an article published in Drug Testing and Analysis during July and August 2014. This research covers essential insights, and its DOI is 10.1002/dta.1620, while it is associated with PMID 24678061.
- Wink CS, Michely JA, Jacobsen-Bauer A, Zapp J, Maurer HH (October 2016): An article in Drug Testing and Analysis from October 2016 presents significant findings related to an undisclosed topic. The article is identified with the DOI 10.1002/dta.1946 and is linked to PMID 26811026.
- Helander A, Beck O, Bäckberg M (June 2015): The clinical implications of intoxications by dissociative new psychoactive substances, specifically diphenidine and methoxphenidine, are addressed in a research article within Clinical Toxicology published in June 2015. The study’s DOI is 10.3109/15563650.2015.1033630, and it corresponds to PMID 25881797 with S2CID 5962038.
- Nancy M. Gray; Brian K. Cheng (6 April 1994): A patent with the reference EP 0346791, concerning the potential use of 1,2-diarylethylamines in the treatment of neurotoxic injury, was filed by G.D. Searle, LLC. The patent, dated April 6, 1994, is accessible via SureChEMBL, with an archival record extending until 2018-09-20, and it was retrieved on 2016-08-08.
- Berger ML, Schweifer A, Rebernik P, Hammerschmidt F (May 2009): In May 2009, a study was published in Bioorganic & Medicinal Chemistry, evaluating the NMDA receptor affinities of 1,2-diphenylethylamine, 1-(1,2-diphenylethyl)piperidine enantiomers, and related compounds. The research article bears the DOI 10.1016/j.bmc.2009.03.025 and has a PMID of 19345586.
- Wallach J, Kavanagh PV, McLaughlin G, Morris N, Power JD, Elliott SP, et al. (May 2015): May 2015 marked the release of a study titled “Preparation and characterization of the ‘research chemical’ diphenidine, its pyrrolidine analogue, and their 2,2-diphenylethyl isomers.” This research is available in Drug Testing and Analysis and is denoted by the DOI 10.1002/dta.1689. An archival PDF version can be accessed until 2020-03-07, and retrieval was documented on 2019-12-10.
- Espinosa L, Itzstein C, Cheynel H, Delmas PD, Chenu C (July 1999): In July 1999, the Journal of Physiology published an article disclosing the expression of active NMDA glutamate receptors in mammalian osteoclasts. The study is linked to the DOI 10.1111/j.1469-7793.1999.0047r.x, and it has PMC 2269403 with PMID 10373688.
- Rogawski MA (September 1993): In September 1993, the therapeutic potential of excitatory amino acid antagonists, encompassing channel blockers and 2,3-benzodiazepines, was the subject of examination in Trends in Pharmacological Sciences. The DOI for this research is 10.1016/0165-6147(93)90005-5, and the PMID is 7504360.
- Kase Y, Yuizono T, Muto M (March 1963): Research from March 1963, appearing in the Journal of Medicinal Chemistry, delves into the role of piperidino groups in antitussive activity. The DOI for this study is 10.1021/jm00338a007, and its PMID is 14188779.
- Cahusac PM, Senok SS, Hitchcock IS, Genever PG, Baumann KI (May 2005): May 2005 saw the exploration of unconventional NMDA receptors in slowly adapting type I mechanoreceptor responses in an article within Neuroscience. The DOI for this work is 10.1016/j.neuroscience.2005.03.018, with a PMID of 15908129 and S2CID 15610561.
- Wallach J, Kang H, Colestock T, Morris H, Bortolotto ZA, Collingridge GL, et al. (17 June 2016): In June 2016, PLOS ONE published “Pharmacological Investigations of the Dissociative ‘Legal Highs’ Diphenidine, Methoxphenidine and Analogues,” marked by the DOI 10.1371/journal.pone.0157021. This research includes PMC 4912077 and PMID 27314670.
- Sahai MA, Davidson C, Dutta N, Opacka-Juffry J (April 2018): In April 2018, Brain Sciences explored the mechanistic aspects of novel psychoactive substances and their interaction with the dopamine transporter. The research is documented with the DOI 10.3390/brainsci8040063, and it has PMC 5924399 and PMID 29642450.
- Wurita A, Hasegawa K, Minakata K, Watanabe K, Suzuki O (August 2014): In August 2014, Forensic Toxicology reported the coexistence of a substantial quantity of the new designer drug diphenidine with a synthetic cannabinoid called 5-fluoro-AB-PINACA in a dubious herbal product. The DOI for this study is 10.1007/s11419-014-0240-y, and it is associated with S2CID 25995354.
- Hasegawa K, Wurita A, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Watanabe K, Suzuki O (January 2015): January 2015 saw the presentation of a postmortem distribution analysis of AB-CHMINACA, 5-fluoro-AMB, and diphenidine in body fluids and solid tissues in a fatal poisoning case. Adipose tissue’s utility in detecting these drugs in unchanged forms is highlighted. The study is available in Forensic Toxicology with the DOI 10.1007/s11419-014-0245-6 and S2CID 11884184.
- Uchiyama N, Shimokawa Y, Kikura-Hanajiri R, Demizu Y, Goda Y, Hakamatsuka T (July 2015): In July 2015, Forensic Toxicology introduced N-OH-EDMA and a cathinone derivative, dimethoxy-α-PHP, which were newly identified in illegal products. The study is accessible with the DOI 10.1007/s11419-015-0268-7 and has PMC 4525202, with PMID 26257833.
- Minakata K, Yamagishi I, Nozawa H, Hasegawa K, Wurita A, Gonmori K, Suzuki M, Watanabe K, Suzuki O (July 2015): In July 2015, Forensic Toxicology utilized MALDI-Q-TOF mass spectrometry to analyze diphenidine and its metabolites in blood and urine. The research is documented with the DOI 10.1007/s11419-015-0273-x and S2CID 44007379.
- Kudo K, Usumoto Y, Kikura-Hanajiri R, Sameshima N, Tsuji A, Ikeda N (September 2015): A fatal case of poisoning related to new cathinone designer drugs, including 4-methoxy PV8, PV9, and 4-methoxy PV9, as well as a dissociative agent known as diphenidine, was reported in Legal Medicine in September 2015. The study is linked to the DOI 10.1016/j.legalmed.2015.06.005 and the PMID 26162997.
- Arsenault D (1 June 2016): On June 1, 2016, Canada Gazette issued regulations amending the Food and Drug Regulations related to Lefetamine, AH-7921, MT-45, and W-18 (Parts G and J). These changes are documented, and the original document is archived until 2017-12-02, with retrieval noted on 2016-11-17.